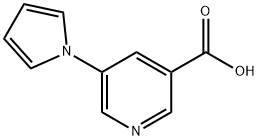
5-(1H-PYRROL-1-YL)NICOTINIC ACID synthesis
- Product Name:5-(1H-PYRROL-1-YL)NICOTINIC ACID
- CAS Number:690632-31-6
- Molecular formula:C10H8N2O2
- Molecular Weight:188.18

690632-31-6
27 suppliers
$90.00/50mg
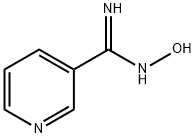
1594-58-7
137 suppliers
$9.00/5g
![3-[3-(3-pyridinyl)-1,2,4-oxadiazol-5-yl]-5-(1H-pyrrol-1-yl)Pyridine](/CAS/20180702/GIF/1033724-02-5.gif)
1033724-02-5
1 suppliers
inquiry
Yield:-
Reaction Conditions:
Stage #1: 5-(1H-pyrrol-1-yl)nicotinic acidwith benzotriazol-1-ol;1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride in N,N-dimethyl-formamide at 20; for 0.333333 h;
Stage #2: N-hydroxy-3-pyridinecarboximidamide in N,N-dimethyl-formamide at 20 - 140; for 8 - 14 h;
Steps:
8
To a solution of 5-(1 H-pyrrol-1 -yl)nicotinic acid (Maybridge, 188 mg, 1 .00 mmol) in dimethylformamide (anhydrous, 5 mL) was added N-(3- methylaminopropyl)-N'-ethylcarbodiimide hydrochloride (EDC) (Aldrich, 192
References:
WO2008/73942,2008,A2 Location in patent:Page/Page column 39; 40

690632-31-6
27 suppliers
$90.00/50mg

1594-58-7
137 suppliers
$9.00/5g
![3-[3-(3-pyridinyl)-1,2,4-oxadiazol-5-yl]-5-(1H-pyrrol-1-yl)Pyridine](/CAS/20180702/GIF/1033724-02-5.gif)
1033724-02-5
1 suppliers
inquiry