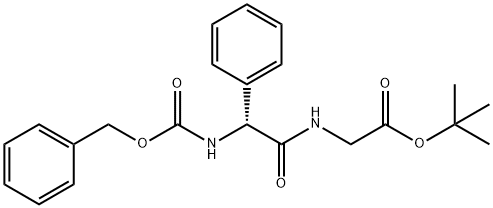
(R)-tert-butyl 2-(2-(benzyloxycarbonylaMino)-2-phenylacetaMido)acetate synthesis
- Product Name:(R)-tert-butyl 2-(2-(benzyloxycarbonylaMino)-2-phenylacetaMido)acetate
- CAS Number:439088-73-0
- Molecular formula:C22H26N2O5
- Molecular Weight:398.45
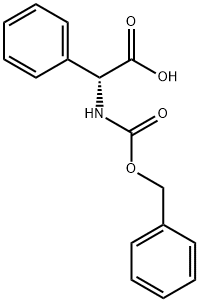
17609-52-8
216 suppliers
$9.00/1g
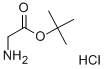
27532-96-3
418 suppliers
$10.00/1G

439088-73-0
22 suppliers
inquiry
Yield:439088-73-0 94%
Reaction Conditions:
Stage #1: Z-D-phenylglycine;glycine tert-butyl ester hydrochloridewith 2,6-dimethylpyridine in dichloromethane at 0; for 0.0833333 h;
Stage #2: with O-(benzotriazol-1-yl)-N,N,N',N'-tetramethyluronium tetrafluoroborate in dichloromethane at 0 - 20; for 5.25 h;
Steps:
3 Method 3; TERT-BUTYLN- ((2R)-2- (BENZVLOXY) CARBONVLLAMINO}-2-PHENYLETHANOYL) GLYCINATE
[(2R)-{[(BENZYLOXY)] carbonyl] amino} (phenyl) acetic acid (Z- (R)-Phg-OH) (10 g, 35.0 mmol) and tert-butylglycine hydrochloride (6.3 g, 37.4 mmol) was dissolved in DCM (200 ml) with 2,6-lutidine (8.2 ml, 70.4 mmol). After stirring 5 minutes at [0°C] TBTU (12.4 g, 38.6 mmol) was added and stirring was continued 1 hours 30 minutes at [0°C] and 3 hours 45 minutes at room temperature. The reaction mixture was washed with water (2 x 100 [ML),] dried (magnesium sulphate) and purified with flash chromatography (DCM: EtOAc 7: [1->5] : 1) to give the title compound (13 g, 94 %). NMR (500 MHz) : 1.45 (s, 9 [H),] 3.84 (d, 1 H), 4.00 (dd, 1 H), 5.10 (m, 2 H), 5. 28 (br s, 1 H), 6.13 (br s, 1 H), 6.23 (br s, 1 H), 7.30-7. 44 (m, 10 H).
References:
WO2004/5247,2004,A1 Location in patent:Page 106