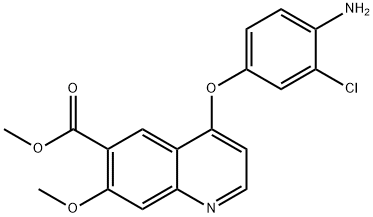
6-Quinolinecarboxylic acid, 4-(4-amino-3-chlorophenoxy)-7-methoxy-, methyl ester synthesis
- Product Name:6-Quinolinecarboxylic acid, 4-(4-amino-3-chlorophenoxy)-7-methoxy-, methyl ester
- CAS Number:417723-07-0
- Molecular formula:C18H15ClN2O4
- Molecular Weight:358.78
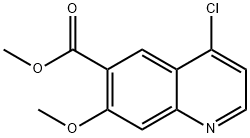
205448-66-4
218 suppliers
$9.00/250mg
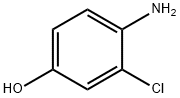
17609-80-2
315 suppliers
$39.00/5g

417723-07-0
19 suppliers
inquiry
Yield:-
Reaction Conditions:
in dimethyl sulfoxide;ethyl acetate;
Steps:
P.395.1 Methyl 4-(4-amino-3-chlorophenoxy)-7-methoxy-6-quinolinecarboxylate
Production Example 395-1 Methyl 4-(4-amino-3-chlorophenoxy)-7-methoxy-6-quinolinecarboxylate After dissolving 4-amino-3-chlorophenol (3.17 g, 22.05 mmol) in dimethylsulfoxide (50 ml), sodium hydride (882 mg, 22.05 mmol) was gradually added at room temperature and the mixture was stirred for 30 minutes. The 4-chloro-7-methoxy-6-methoxycarbonylquinoline (3.70 g, 14.7 mmol) described in WO0050405 was added, and the mixture was heated at 100° C. for 3 hours. After standing to cool to room temperature, the reaction solution was distributed between ethyl acetate and water, and the organic layer was washed with water and saturated brine and dried over anhydrous sodium sulfate. The solvent was distilled off, silica gel column chromatography (eluent-ethyl acetate) was performed, the fraction containing the target substance was concentrated, suspended in ethyl acetate and diluted with hexane, and the crystals were filtered out and blow-dried to obtain the title compound (3.092 g, 8.62 mmol, 57.4%) as light brown crystals. 1H-NMR Spectrum (CDCl3) δ (ppm): 3.98 (3H, s), 4.06 (3H, s), 4.12 (2H, s), 6.44 (1H, d, J=5.2 Hz), 6.86 (1H, d, J=8.8 Hz), 6.95 (1H, dd, J=2.8, 8.8 Hz), 7.16 (1H, d, J=2.8 Hz), 7.49 (1H, s), 8.64 (1H, d, J=5.2 Hz), 8.80 (1H, s).
References:
US2004/53908,2004,A1

205448-66-4
218 suppliers
$9.00/250mg

417723-07-0
19 suppliers
inquiry
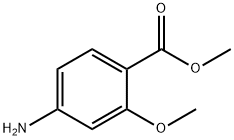
27492-84-8
315 suppliers
$6.00/5g

417723-07-0
19 suppliers
inquiry
![4-[(2,2-Dimethyl-4,6-dioxo-[1,3]dioxan-5-ylidenemethyl)-amino]-2-methoxy-benzoic acid methyl ester](/CAS/20180703/GIF/205448-64-2.gif)
205448-64-2
79 suppliers
$25.00/250mg

417723-07-0
19 suppliers
inquiry
205448-65-3
163 suppliers
$13.00/100mg

417723-07-0
19 suppliers
inquiry