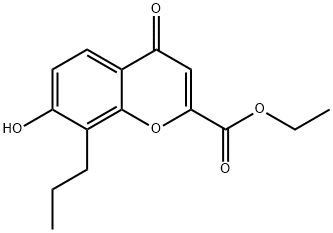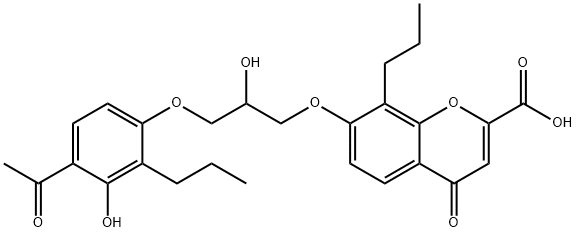
7-[3-(4-ACETYL-3-HYDROXY-2-PROPYLPHENOXY)-2-HYDROXYPROPOXY]-4-OXO-8-PROPYL-4H-1-BENZOPYRAN-2-CARBOXYLIC ACID SODIUM SALT synthesis
- Product Name:7-[3-(4-ACETYL-3-HYDROXY-2-PROPYLPHENOXY)-2-HYDROXYPROPOXY]-4-OXO-8-PROPYL-4H-1-BENZOPYRAN-2-CARBOXYLIC ACID SODIUM SALT
- CAS Number:40785-97-5
- Molecular formula:C27H30O9
- Molecular Weight:498.52
Yield:-
Reaction Conditions:
with sodium hydroxide;N-benzyl-trimethylammonium hydroxide;sodium hydrogencarbonate in methanol;ethanol;water;ethyl acetate;N,N-dimethyl-formamide;
Steps:
1.F Step F:
Step F: 7-[3-(4-Acetyl-3-hydroxy-2-propylphenoxy)-2 -hydroxypropoxy]-4-oxo-8-propyl-4H-1-benzopyran-2-carboxylic Acid Dissolve 26 gm of 4-(2,3-epoxypropoxy)-2-hydroxy-3-propylacetophenone and 26 gm of ethyl 7-hydroxy-8-propyl-4-oxo-4H-1-benzopyran-2-carboxylate in 150 ml of N,N-dimethylformamide and add 5 drops of Triton B (benzyltrimethylammonium hydroxide 40% in methanol). Heat at 140°-150° C. under nitrogen for 4 hours. Cool the mixture and pour into 1200 ml of water. Extract three times with 400 ml of ethyl acetate. Wash the combined organic fractions with water, then twice with 250 ml of 2% aqueous sodium hydroxide and again with water. Dry over sodium sulfate and strip to a residue. Chromatograph the residue over silica gel (1200 gm), eluding with a mixture of toluene-ethyl acetate (10:1). Dissolve the ester product (30 gm) in 1 liter of ethanol and add 30 gm of sodium bicarbonate and 100 ml of water. Reflux for 2 hours. Evaporate most of the solvent and partition the residue between ethyl acetate and 5% aqueous sodium hydroxide. Acidify the aqueous fraction and extract into ethyl acetate. Strip the extract to a residue and triturate the residue in 300 ml of ether. Recover the solids by filtration. (mp 199°-201° C.).
References:
US4252818,1981,A
![1-[2-HYDROXY-4-(OXIRAN-2-YLMETHOXY)-3-PROPYLPHENYL]ETHAN-1-ONE](/CAS/GIF/57161-85-0.gif)