4-VINYLBIPHENYL synthesis
- Product Name:4-VINYLBIPHENYL
- CAS Number:2350-89-2
- Molecular formula:C14H12
- Molecular Weight:180.25
Yield:2350-89-2 99%
Reaction Conditions:
with N-Methyldicyclohexylamine;palladium diacetate;tri tert-butylphosphoniumtetrafluoroborate in methanol;toluene at 120; under 11251.1 Torr;Flow reactor;
Steps:
10 4-PhenyIstyrene (32)
4-lodobiphenyl (1 .5 mmol), Cy2NMe (1 .2 eq), Pd(OAc)2 (1 mol%), tBuPH.BF4 (2 mol%) were dissolved in PhMe/MeOH (5 mL, 9:1). The reaction mixture was injected into a UniqsisTM Flowsyn reactor (Uniqsis Ltd. Shepreth, Cambridgeshire, UK) via a 5 mL PEEK injectionloop. The reaction plug was pumped at 1.0 mL/min (using PhMe/MeOH (9:1) as stock solvent) through a tube-in-tube gas reactor (1 .5 m AF2400 obtained from Biogeneral Inc.:http://www.biogeneral.com/teflon.html) pressurised with ethylene (15 bar) followed by a 20 mL PTFE reaction coil at 120 °C. The exiting reaction stream was passed through an Omnifit column ( Kinesis Ltd., St. Neots, Cambridgeshire, UK) containing a mixture ofQP-TU and QP-SA followed by a BPR (20 bar). A fraction (containing the reaction plug and any dispersion) was collected and flushed with argon. The solvent was removed from the product fraction under vacuum to provide 4-phenylstyrene as a colourless solid (268 mg, 1.5 mmol, 99%). The title compound 32 was obtained as colourlesscrystalline (plates) solid; mpll8-120 °C; 1H NMR (400MHz, CDCI3) O 5.29 (d, J=11.0 Hz, 1H, H6a), 5.81 (d,J17.6 Hz, 1H, H6b), 6.78 (dd, J17.6, 11.0 Hz, 1H, H5),7.36 (t, J7.5 Hz, 1H, H4’), 7.45 (t, J7.5 Hz, 2H, H3’),7.50 (d, J=8.2 Hz, 2H, H3), 7.59 (d, J8.0 Hz, 2H, H2), 7.62 (d, J=7.8 Hz, 2H, H2’), 13C NMR (101 MHz, CDCI3) O 113.8 (CH2, C6), 126.6 (2CH, C3), 126.9 (2CH, C2’), 127.2 (2CH, C2), 127.3 (CH, C4’), 128.7 (2CH, C3’), 136.4 (C, C5), 136.6 (C, C4), 140.5 (C, Cl), 140.7 (C, Cl’), IR (vmaxcm1) 3034 (C-H aromatic), 1626 (C=C alkenyl), 1483 (C=C aromatic), HRMS10 Elk: m/z [M+H] Calcd for C14H13: Expected: 181.1012. Found: 181.1012.
References:
WO2017/51326,2017,A1 Location in patent:Page/Page column 50; 51
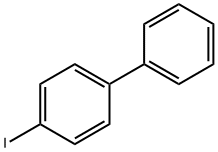
3562-73-0
97 suppliers
$12.00/250mg

2350-89-2
102 suppliers
$15.00/1g
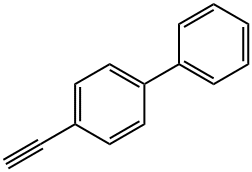
29079-00-3
131 suppliers
$9.00/250mg

2350-89-2
102 suppliers
$15.00/1g
37729-18-3
50 suppliers
$112.00/500mg

2350-89-2
102 suppliers
$15.00/1g
1779-49-3
608 suppliers
$5.00/10g
3218-36-8
336 suppliers
$6.00/5g

2350-89-2
102 suppliers
$15.00/1g