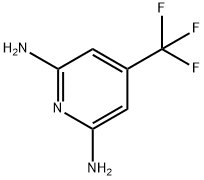
4-Trifluoromethyl-2,6-pyridinediamine synthesis
- Product Name:4-Trifluoromethyl-2,6-pyridinediamine
- CAS Number:130171-52-7
- Molecular formula:C6H6F3N3
- Molecular Weight:177.13
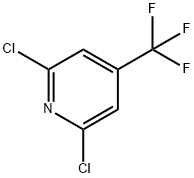
39890-98-7
188 suppliers
$13.00/1g

130171-52-7
19 suppliers
$65.00/10mg
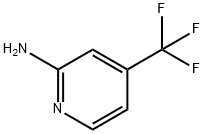
106447-97-6
348 suppliers
$11.00/1g
Yield:-
Reaction Conditions:
Stage #1: 2,6-dichloro-4-(trifluoromethyl)pyridinewith ammonia in water at 150; for 15 h;autoclave;
Stage #2: with hydrogen;5%-palladium/activated carbon in water at 100; under 13501.4 Torr; for 3 h;
Steps:
2
Comparative Example 2In an autoclave (100 mL), 5 g (0.023 moles) of 2,6,4-DCTF and 15.3 mL (0.22 moles) of 28 % ammonia water were heated to 150 °C with stirring to effect reaction. After about 15 hours, the autoclave was cooled to from 30 to 40 °C. 150 mg of 5 % Pd/C (54 % wet, 0.038 mmoles) was added thereto, hydrogen was filled up to 1.8 MPa, followed by heating to 100°C with stirring to effect reaction. After about 3 hours, the autoclave was cooled to from 30 to 40 °C, followed by filtration through Celite. Water was added to the filtrate; the extraction with ethyl acetate was conducted three times; and an organic layer was washed with saturated saline and then dried over sodium sulfate. The organic layer was concentrated under reduced pressure, and after adding n-hexane, followed by concentration under reduced pressure. A deposited crystal was dried under reduced pressure, thereby obtaining 3 g of 2,4-ATF as a pale ocher crystal (yield (crude): 79.9 %).As for each of Examples 2, 3 and 4 and Comparative Example 2, the obtained crystal was analyzed by high performance liquid chromatography (HPLC). HPLC-PA % of each of 2,6,4-ACTF as an intermediate product, 2,4-ATF as a desired compound, and 2,6,4-DATF as a by-product and the yield of 2,4-ATF are described in Table 2.
References:
EP2527327,2012,A1 Location in patent:Page/Page column 6-7

39890-98-7
188 suppliers
$13.00/1g

130171-52-7
19 suppliers
$65.00/10mg
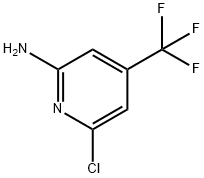
34486-23-2
67 suppliers
$40.00/100mg

130171-52-7
19 suppliers
$65.00/10mg