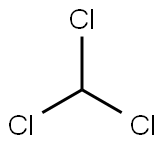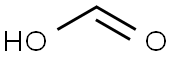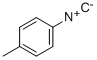
4-TOLYLISOCYANIDE synthesis
- Product Name:4-TOLYLISOCYANIDE
- CAS Number:7175-47-5
- Molecular formula:C8H7N
- Molecular Weight:117.1479
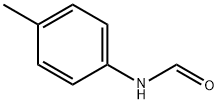
3085-54-9
59 suppliers
$39.89/250mg

7175-47-5
9 suppliers
inquiry
Yield:7175-47-5 95%
Reaction Conditions:
with diisopropylamine;trichlorophosphate in dichloromethane at 0 - 20;Inert atmosphere;
Steps:
Representative procedure for the synthesis of isonitriles: Compound (2g)
To a stirring 0 °C mixture of DIPA (2.7 equiv, 3.9 mL) and N-(3-bromophenyl)formamide (2.06 g) in DCM (0.9 M), as added POCl3 (1.1 equiv, 1.06 mL) dropwise under argon. After 5 min at 0°C and 15 min at room temperature, 10 mL water was added and mixed vigorously until the organic layer became clear. The organic layer was separated, loaded onto a short silica gel flash column, and eluted with 4:1 hexanes:ethyl acetate to give 1.53 g (81%) of a foul-smelling yellow liquid which became dark green after drying in vacuo 10 min.
References:
Polisar, Jason G.;Li, Ling;Norton, Jack R. [Tetrahedron Letters,2011,vol. 52,# 23,p. 2933 - 2934] Location in patent:supporting information; experimental part
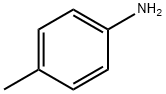
106-49-0
398 suppliers
$5.00/5G

7175-47-5
9 suppliers
inquiry
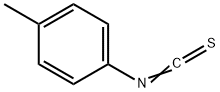
622-59-3
108 suppliers
$27.00/1g

7175-47-5
9 suppliers
inquiry