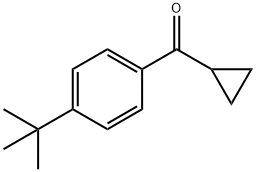
4-tert-Butylphenyl cyclopropyl ketone synthesis
- Product Name:4-tert-Butylphenyl cyclopropyl ketone
- CAS Number:38675-79-5
- Molecular formula:C14H18O
- Molecular Weight:202.29
925430-09-7
0 suppliers
inquiry
201230-82-2
1 suppliers
inquiry
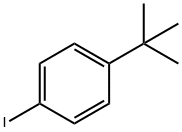
35779-04-5
194 suppliers
$6.00/1g

38675-79-5
41 suppliers
inquiry
Yield:38675-79-5 23 mg
Reaction Conditions:
with (η3-allyl)(N,N'-bis(2,6-diisopropylphenyl)-4,5-dihydroimidazol-2-ylidene)chloropalladium(II);sodium carbonate;lithium chloride in N,N-dimethyl-formamide at 80; under 760.051 Torr; for 16 h;Inert atmosphere;Sealed tube;Temperature;
Steps:
5. General procedure for the carbonylative cross-coupling
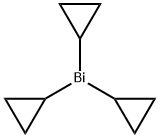
General procedure: Method A: A sealed tube equiped with a magnetic stirring bar was charged with the arylhalide (1) or (5) (1.0 equiv), sodium carbonate (2.0 equiv), anhydrous lithium chloride (2.0equiv) and (SIPr)Pd(allyl)Cl (0.05 equiv). Tricyclopropylbismuth (2a) (1.0 equiv), preparedas described above, was dissolved in anhydrous DMF (0.1 M) under argon and was addedinto the sealed tube. Carbon monoxide was bubbled in the reaction mixture for 45 seconds,then the tube was sealed and heated at 40 °C for 16 hours. The reaction mixture was cooledto room temperature, transferred in a separatory funnel containing 20 mL of an aq. sat.NaHCO3 solution and was extracted with EtOAc (3 x 20 mL). The combined organic layerswere washed with brine (30 mL), dried over anhydrous Na2SO4 and concentrated underreduced pressure. The residue was purified by flash column chromatography using theindicated solvent system to afford the desired aryl cyclopropyl ketone (3) or (6).Method B: Same as method A except that 1.5 equivalents of tricyclopropylbismuth 2ainstead of 1.0 equivalent and 0.1 equivalents of (SIPr)Pd(allyl)Cl instead of 0.05 equivalentswere used and that the reaction was heated at 80 C instead of 40 C.
References:
Benoit, Emeline;Dansereau, Julien;Gagnon, Alexandre [Synlett,2017,vol. 28,# 20,p. 2833 - 2838] Location in patent:supporting information
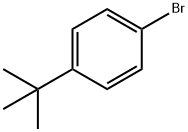
3972-65-4
320 suppliers
$8.00/10g
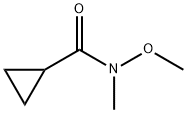
147356-78-3
88 suppliers
$25.00/1g

38675-79-5
41 suppliers
inquiry
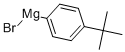
63488-10-8
73 suppliers
$152.00/50mL

38675-79-5
41 suppliers
inquiry
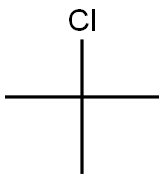
507-20-0
365 suppliers
$16.00/25mL

3481-02-5
114 suppliers
$14.00/1g

38675-79-5
41 suppliers
inquiry