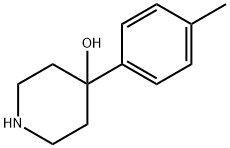
4-(p-tolyl)piperidin-4-ol synthesis
- Product Name:4-(p-tolyl)piperidin-4-ol
- CAS Number:57988-60-0
- Molecular formula:C12H17NO
- Molecular Weight:191.27
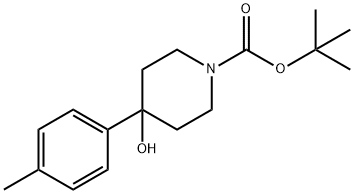
1260882-14-1
6 suppliers
inquiry

57988-60-0
13 suppliers
$45.00/50mg
Yield:57988-60-0 40%
Reaction Conditions:
with hydrogenchloride in ethyl acetate at 20;
Steps:
4-p-tolylpiperidin-4-ol(11f)
A solution of 10f (4.157g) and 3M HCl (10mL) in EtOAc (50mL) was stirred at room temperature. The reaction was neutralized with saturated NaOH, and the mixture was extracted with CH2Cl2 (3x30mL), washed with saturated NaCl solution and dried with Na2SO4. Filtration, evaporation in vacuo, and purification by flash chromatography (DCM: MeOH = 50:1-5:1) afforded 1.13g (40%) as a solid. Mp: 146-147 °C. 1H NMR (300 MHz, CDCl3) δ 7.36 (d, J = 8.4 Hz, 2H), 7.14 (d, J = 8.4 Hz, 2H), 4.51 (m, 2H), 3.19-3.24 (m, 2H), 3.09 (m, 2H), 2.32 (s, 3H), 2.15-2.16 (m, 2H), 1.76-1.80 (m, 2H). LRESI-MS m/z: 192.0[M+H]+, 174.0[M+H-H20]+.
References:
Chen, Gang;Xia, Hongguang;Cai, Yu;Ma, Dawei;Yuan, Junying;Yuan, Chengye [Bioorganic and Medicinal Chemistry Letters,2011,vol. 21,# 1,p. 234 - 239] Location in patent:supporting information; experimental part
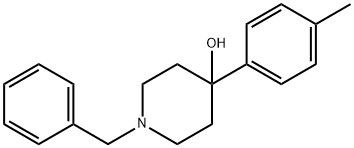
13299-35-9
3 suppliers
inquiry

57988-60-0
13 suppliers
$45.00/50mg

13299-35-9
3 suppliers
inquiry
7440-05-3
483 suppliers
$17.69/1G

57988-60-0
13 suppliers
$45.00/50mg
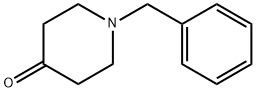
3612-20-2
488 suppliers
$6.00/10g

57988-60-0
13 suppliers
$45.00/50mg
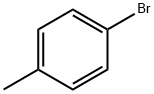
106-38-7
401 suppliers
$14.00/5g

57988-60-0
13 suppliers
$45.00/50mg