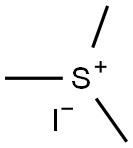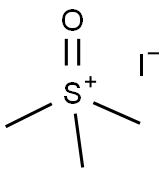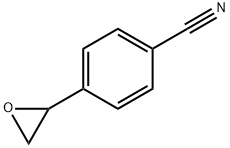
4-oxiran-2-ylbenzonitrile synthesis
- Product Name:4-oxiran-2-ylbenzonitrile
- CAS Number:52695-39-3
- Molecular formula:C9H7NO
- Molecular Weight:145.16
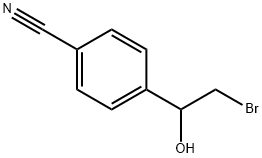
85554-13-8
3 suppliers
inquiry

52695-39-3
18 suppliers
inquiry
Yield:52695-39-3 289 mg
Reaction Conditions:
with potassium hydroxide in diethyl ether;water at 20;
Steps:
4-Cyanostyrene oxide 1n
Epoxide 1n was prepared according to a modified literature procedure.14 Sodium borohydride (106mg, 2.7mmol) was added to a solution of 2-bromo-1-(4-cyanophenyl)-ethanone (568mg, 2.54mmol) in methanol (7mL) and stirred at 0°C. After 1.5h, water (10mL) was added and reaction mixture was stirred for 1h at room temperature. Reaction mixture was extracted with dichloromethane (3×10mL), combined organic layers were dried over Na2SO4, filtered and evaporated under reduced pressure. The dry residue was dissolved in a small amount of diethyl ether, following by addition of potassium hydroxide (15mL, 1M) and the reaction mixture was stirred over night at room temperature. Reaction mixture was extracted with dichloromethane (4×10mL), combined organic layers were washed with brine, dried over Na2SO4, filtered and evaporated under reduced pressure. Column chromatography (SiO2; hexane-ethyl acetate, 7:3) furnished the pure epoxide 1n (289mg, 78%) as a colourless liquid. dH (300MHz, CDCl3) 2.74 (1H, dd, J1 5.5Hz, J2 2.5Hz), 3.18 (1H, dd, J1 5.5Hz, J2 4.0Hz), 3.88-3.90 (1H, m), 7.37 (2H, d, J 8.5Hz), 8.62 (2H, d, J 8.5Hz); dC (75MHz, CDCl3) 51.5, 51.6, 112.0, 118.6, 126.1, 132.3, 143.3
References:
Mikleu?evi?, Ana;Primo?i?, Ines;Hrenar, Tomica;Salopek-Sondi, Branka;Tang, Lixia;Elenkov, Maja Majeri? [Tetrahedron Asymmetry,2016,vol. 27,# 19,p. 930 - 935]
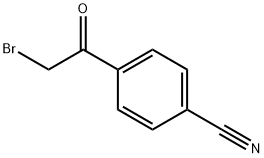
20099-89-2
421 suppliers
$10.00/1g

52695-39-3
18 suppliers
inquiry
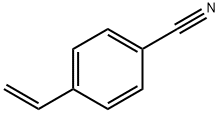
3435-51-6
60 suppliers
$29.00/100mg

52695-39-3
18 suppliers
inquiry