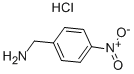
4-Nitrobenzylamine hydrochloride synthesis
- Product Name:4-Nitrobenzylamine hydrochloride
- CAS Number:18600-42-5
- Molecular formula:C7H9ClN2O2
- Molecular Weight:188.61
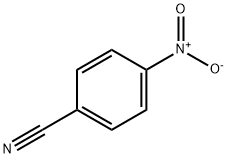
619-72-7
368 suppliers
$11.00/1g

18600-42-5
142 suppliers
$9.00/250mg
Yield:18600-42-5 90%
Reaction Conditions:
Stage #1:4-nitrobenzonitrile with C46H178O41Si42;titanium(IV)isopropoxide in toluene at 60; for 9 h;Inert atmosphere;
Stage #2: with hydrogenchloride;water in toluene at 20;Inert atmosphere;chemoselective reaction;Reagent/catalyst;
Steps:
3 4.2. General procedure for the reduction of nitriles
General procedure: To a nitrogen purged screw-caped vial containing 1a (1.0 g, 5.1 mmol, 1.0 equiv) in 6.0 mL of toluene were added TMDS (900 μL, 5.1 mmol, 1.0 equiv) or PMHS (610 μL, 10.2 mmol, 2.0 equiv) and Ti(Oi-Pr)4 (1.5 mL, 5.1 mmol, 1.0 equiv) at rt. The mixture was then heated at 60 °C for 24 h (the colorless solution turned into black and the conversion of the substrate can be followed up by TLC and/or 1H NMR). After cooling to rt, the clear solution was acidified using aqueous 1 M HCl (7.7 mL, 1.5 equiv) and the crude mixture was concentrated under reduced pressure. The resulting solid was filtered, washed with pentane (3*50 mL), and dissolved in ethanol. The filtrate was finally concentrated under reduced pressure affording the amine 2a as a hydrochloride salt.
References:
Laval, Stéphane;Dayoub, Wissam;Pehlivan, Leyla;Métay, Estelle;Favre-Reguillon, Alain;Delbrayelle, Dominique;Mignani, Gérard;Lemaire, Marc [Tetrahedron,2014,vol. 70,# 4,p. 975 - 983] Location in patent:supporting information
619-80-7
5 suppliers
$13.00/5g

18600-42-5
142 suppliers
$9.00/250mg
17271-88-4
11 suppliers
inquiry

18600-42-5
142 suppliers
$9.00/250mg
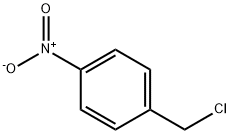
100-14-1
3 suppliers
$10.00/5g

18600-42-5
142 suppliers
$9.00/250mg
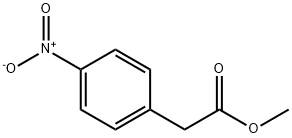
2945-08-6
61 suppliers
inquiry

18600-42-5
142 suppliers
$9.00/250mg