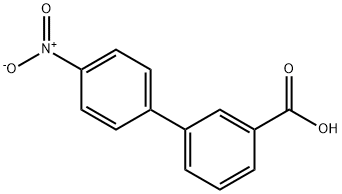
4'-Nitro-3-biphenylcarboxylic acid synthesis
- Product Name:4'-Nitro-3-biphenylcarboxylic acid
- CAS Number:729-01-1
- Molecular formula:C13H9NO4
- Molecular Weight:243.21
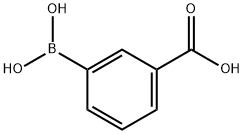
25487-66-5
470 suppliers
$6.00/5g
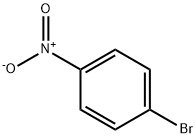
586-78-7
3 suppliers
$14.00/5g

729-01-1
16 suppliers
$134.75/0.25g
Yield:729-01-1 100%
Reaction Conditions:
with potassium carbonate;tetrakis(triphenylphosphine) palladium(0) in ethanol;water;toluene; for 48 h;Heating / reflux;Suzuki Coupling;
Steps:
6.a
1-Bromo-4-nitrobenzene (1.95g, 9. 6mmol) and 3- carboxybenzeneboronic acid (1. 8g, 10.8mmol, l. lequiv. ) were dissolved in a mixture of toluene (40mL), ethanol (40mL) and water (5mL) and potassium carbonate (4. 1g, 29.3mmol, 3. Oequiv.) was added. The flask was purged with nitrogen gas then palladium tetrakis (triphenylphosphine) (0.2g) was added and the mixture heated at reflux for 48 hours. The reaction mixture was cooled to room temperature and diluted with ethyl acetate (lOOmL) and extracted with water (3 x 50mL). The aqueous extracts were combined and washed with dichloromethane (3 x 50mL). The aqueous fraction was acidified (pH 1-2) with concentrated hydrochloric acid to give an off white precipitate, which was collected on a filter and dried under vacuum to give a white solid 14,2. 34g, (100%). H NMR (d6-DMSO) 5 13.20 (1H, bs, OH), 8.36-8. 29 (3H, m, Ar-H), 8.07-8. 01 (4H, m, Ar-H), 7.69 (1H, d, J = 7.8Hz, H-6) ; lac NMR (d6- DMSO) 5 166.9, 146.9, 145.6, 138.2, 131.7 (CH), 131.6, 129.6 (CH), 128.0 (CH), 127.8 (CH), 124.1 ; LCMS RT = 3.28 min, (M--l) = 242.
References:
WO2005/85177,2005,A2 Location in patent:Page/Page column 48
![Methyl 4'-nitro-[1,1'-biphenyl]-3-carboxylate](/CAS/GIF/107558-26-9.gif)
107558-26-9
13 suppliers
$60.00/50mg

729-01-1
16 suppliers
$134.75/0.25g
952-21-6
20 suppliers
$45.00/50mg

729-01-1
16 suppliers
$134.75/0.25g
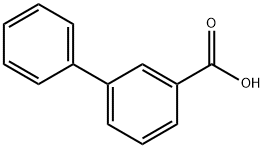
716-76-7
176 suppliers
$6.00/250mg

729-01-1
16 suppliers
$134.75/0.25g
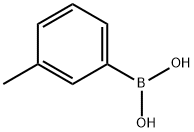
17933-03-8
363 suppliers
$5.00/1g

729-01-1
16 suppliers
$134.75/0.25g