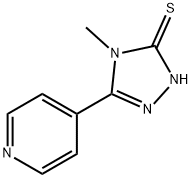
4-METHYL-5-PYRIDIN-4-YL-4H-[1,2,4]TRIAZOLE-3-THIOL synthesis
- Product Name:4-METHYL-5-PYRIDIN-4-YL-4H-[1,2,4]TRIAZOLE-3-THIOL
- CAS Number:3652-32-2
- Molecular formula:C8H8N4S
- Molecular Weight:192.24
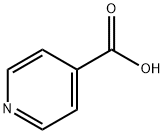
55-22-1
634 suppliers
$5.00/5g
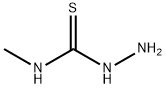
6610-29-3
212 suppliers
$19.00/1 g
![4-METHYL-5-PYRIDIN-4-YL-4H-[1,2,4]TRIAZOLE-3-THIOL](/CAS/GIF/3652-32-2.gif)
3652-32-2
54 suppliers
$58.00/250mg
Yield: 84%
Reaction Conditions:
with 2,4,6-tripropyl-1,3,5,2,4,6-trioxatriphosphinane-2,4,6-trioxide;N-ethyl-N,N-diisopropylamine in ethyl acetate;N,N-dimethyl-formamide at 0 - 20;
Steps:
30 Preparation 30: 4-methyl-5 -(pyridin-4-yl)-4H- 1 ,2,4-triazole-3 -thiol
To a solution of pyridine-4-carboxylic acid (1.0 g, 8.12 mmol) and 4-methyl-3- thiosemicarbazide (0.94 g, 8.93 mmol) in DMF (4 mL), DIPEA (2.6 mL, 14.62 mmol) wasadded. The stirred mixture was cooled to 0 °C then T3P (50% wt /EA) (7.3 mL, 12.18 mmol) was added portion-wise. The ice-bath was removed and the resulting reaction mixture was shaken at RT in a PLS apparatus on. Aqueous 4 M NaOH solution was added (resulting in pH8) and the two resulting phases were separated (the upper organic layer was eliminated). Additional 4 M NaOH was added up to pH 11 then the mixture was heated to 70 °C and stirred for 40 mm.The solution was cooled to RT and 37% HC1 was slowly added until pH 5 and a precipitate started to form. The mixture was stirred for 10 mm then filtered. The solid was washed with water and dried under vacuum at 45 °C ON affording 1.31 g of title compound (p30, y84%). MS (m/z): 193.1[MH]t
References:
INDIVIOR UK LIMITED;CREMONESI, Susanna;MICHELI, Fabrizio;SEMERARO, Teresa;TARSI, Luca WO2017/21920, 2017, A1 Location in patent:Paragraph 0555; 0556
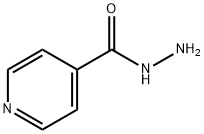
54-85-3
693 suppliers
$6.00/10g
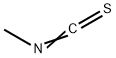
556-61-6
182 suppliers
$19.00/5g
![4-METHYL-5-PYRIDIN-4-YL-4H-[1,2,4]TRIAZOLE-3-THIOL](/CAS/GIF/3652-32-2.gif)
3652-32-2
54 suppliers
$58.00/250mg
![4-Pyridinecarboxylicacid, 2-[(methylamino)thioxomethyl]hydrazide](/CAS/GIF/4406-96-6.gif)
4406-96-6
16 suppliers
inquiry
![4-METHYL-5-PYRIDIN-4-YL-4H-[1,2,4]TRIAZOLE-3-THIOL](/CAS/GIF/3652-32-2.gif)
3652-32-2
54 suppliers
$58.00/250mg

54-85-3
693 suppliers
$6.00/10g
![4-METHYL-5-PYRIDIN-4-YL-4H-[1,2,4]TRIAZOLE-3-THIOL](/CAS/GIF/3652-32-2.gif)
3652-32-2
54 suppliers
$58.00/250mg
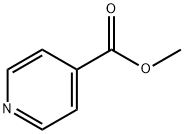
2459-09-8
296 suppliers
$7.00/10g
![4-METHYL-5-PYRIDIN-4-YL-4H-[1,2,4]TRIAZOLE-3-THIOL](/CAS/GIF/3652-32-2.gif)
3652-32-2
54 suppliers
$58.00/250mg