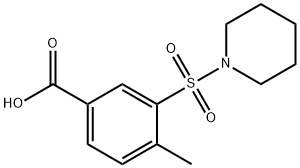
4-METHYL-3-(PIPERIDINE-1-SULFONYL)BENZOIC ACID synthesis
- Product Name:4-METHYL-3-(PIPERIDINE-1-SULFONYL)BENZOIC ACID
- CAS Number:300383-07-7
- Molecular formula:C13H17NO4S
- Molecular Weight:283.34
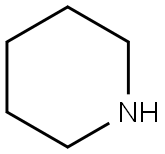
110-89-4
3 suppliers
$15.96/100ML
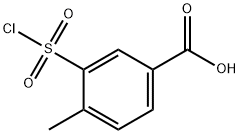
2548-29-0
78 suppliers
$24.00/1g

300383-07-7
33 suppliers
$270.00/5g
Yield:300383-07-7 52%
Reaction Conditions:
in acetonitrile at 0 - 20;
Steps:
A.2; F.2
Step 2: Synthesis of 4-Methyl-3-(piperidine-l-sulfonyl)-benzoic acid; To a suspension of 8 g (34.2 mmol) 3-chlorosulfonyl-4-methyl-benzoic acid in acetonitrile (160 mL) was added dropwise 10.1 mL (103 mmol, 3 equ.) of piperidine at 0 0C. The reaction was stirred at 0 0C for 0.5 h, then allowed to warm to room temperature and stirred until completion. The reaction was concentrated under reduced pressure to l/8th of the volume. The residue was dissolved in IM aqueous NaOH solution (50 mL) and washed with TBME (2 x 50 mL). The basic aqueous layer was cooled in an ice-bath and acidified with 2M aqueous HCl solution to pH 1-2. This acidic aqueous layer was then extracted with TBME (3 x 50 mL). The combined organic extracts were washed with brine (50 mL), dried over Na2SO4, filtered and concentrated under reduced pressure to afford 5.0 g of 4-methyl-3 -(piperidine- 1 -sulfonyl)-benzoic acid.; Step 2: Synthesis of 4-Methyl-3-(piperidine-l-sulfonyl)-benzoic acid;
References:
WO2008/98025,2008,A1 Location in patent:Page/Page column 20; 21; 49-50
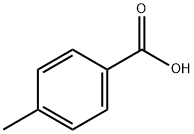
99-94-5
592 suppliers
$9.00/1g

300383-07-7
33 suppliers
$270.00/5g