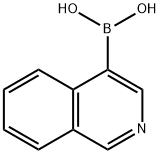
4-Isoquinolineboronic acid synthesis
- Product Name:4-Isoquinolineboronic acid
- CAS Number:192182-56-2
- Molecular formula:C9H8BNO2
- Molecular Weight:172.98
1532-97-4
351 suppliers
$14.00/10g
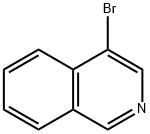
5419-55-6
376 suppliers
$12.00/5g

192182-56-2
185 suppliers
$38.88/250mgs:
Yield:-
Reaction Conditions:
with n-butyllithium in tetrahydrofuran;hexane;ethyl acetate
Steps:
11 3-(isoquinolin-4-yl)-8-methyl-8-azabicyclo[3.2.1]octane
EXAMPLE 11 3-(isoquinolin-4-yl)-8-methyl-8-azabicyclo[3.2.1]octane A solution of 4-bromoisoquinoline in tetrahydrofuran is cooled to -78° C. To this solution is added dropwise n-butyllithium (1.6 M in hexane), and the resultant solu-tion is stirred for 30 minutes. To this solution is then added dropwise triisopropylborate and the reaction mixture is then stirred for 18 hours at room temperature. The reaction mixture is then partitioned between ethyl acetate and saturated aqueous sodium chloride. The phases are separated and the aqueous phase extracted well with ethyl acetate. The combined organic phases are washed with saturated aqueous sodium chloride, dried over sodium sulfate and concentrated under reduced pressure. The residue is sonicated in a mixture of hexane:ethyl acetate. The resulting suspension is filtered to provide isoquinolin-4-ylboronic acid.
References:
Eli Lilly and Company US6107307, 2000, A