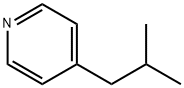
4-ISOBUTYL-PYRIDINE synthesis
- Product Name:4-ISOBUTYL-PYRIDINE
- CAS Number:4810-79-1
- Molecular formula:C9H13N
- Molecular Weight:135.21
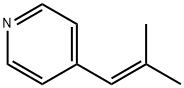
6760-50-5
0 suppliers
inquiry

4810-79-1
23 suppliers
$715.51/1G
Yield:4810-79-1 90%
Reaction Conditions:
with hydrogen;palladium 10% on activated carbon under 760.051 Torr;
Steps:
54
4-Isobutyl-2-cyanopyridine is prepared as follows. To isobutyltriphenylphosphonium iodide (Aldrich) (8 g, 18.5 mmol) in THF (20 mL) at 0 °C, potassium-tert-butoxide (1.8 g, 16 mmol) was added, stirred at room temperature for 1 hr. pyridine-4-carboxlaldehyde (1 g, 9.3 mmol) was added, stirred at room temperature for overnight. Reaction mixture was then poured to water (100 mL) and extracted with EtOAc (100 mL). The product obtained on removal of solvent was purified by column chromatography using 40% EtOAc in hexane as eluent (1.05 g, [84%).] To the resulting product (4.2 g, 31.5 mmol), 10% Pd/C (0.4 g) was added and hydrogenated at 1 atm pressure overnight. Removal of solvent and purification on column chromatography using 30% EtOAc in hexanes resulted in 4-isobutylpyridine (3.8 g, [90%).] [0468] The intermediate, 4-isobutylpyridine-2-carboxylic acid, compound lOb, [(R9 =ISOBUTYL)] was made by employing general Method P. To 4-isobutylpyridine (2 g, 14.8 mmol) in acetic acid (20 mL), hydrogen peroxide (3. [35] g, [30] %, 30 mmol) was added and refluxed overnight. After removing the solvent, the residue was dissolved in DCM dried over magnesium sulfate and taken as such for the next step. To the compound in DCM (10 [ML)] trimethylsilyl cyanide (1.73 g, 17.4 mmol) and dimethylcarbonyl chloride (1.89 g, 17.4 mmol) was added and stirred at room temperature for 24 hours. Aqueous potassium bicarbonate (100 mL, 10%) was added and extracted twice with DCM (50 mL each). The crude product obtained on removal of solvent was taken in HCl [(6N,] 100 mL) and refluxed for 24 hours. Removal of acid and purification of crude product by column chromatography using 30% MeOH in DCM resulted in acid 10b (R9 =isobutyl) (1.5 g, [100%).] [[0469] 1H] NMR (300 MHz, CD30D) 8 8.78 (d, [J=5.] 7,1), 8. 44 (s, 1), 8.13 (d, [J=5.] 7,1), 2.92 (d, [J=7.] 5,1), 2.15 (m, 1), 0.98 (d, [J=6.] 6,6). MS (ES-): [178] [(M-1).] [[0470]] To the amine 2b (Rl=Me, [R=ME)] (200 mg, 0.79 mmol) in DMF [(2ML),] TEA (161 mg, 1.6 mmol), BSTFA (614 mg, 2.4 mmol) was added at 0 [°C] and stirred at room temperature for 1.5 hr. Acid 10b (R9 = isobutyl) (214 mg, 1.2 mmol) and HATU (368 mg, 1.2 mmol) was added and let stirred at room temperature for 4 hours. DMF was removed and the residue was extracted with EtOAc (50 mL), washed with sodium bicarbonate (10%, 50 mL), brine (50 mL) and dried over magnesium sulfate. The product obtained on removal of solvent was dissolved in methanol (10 mL) and treated with NR-50 resin (300 mg) for 3 hr. After filtering the resin, methanol was removed to obtain the crude product. It was then purified on silica gel column chromatography using 3% MeOH in DCM to obtain compound [LLB (RL=ME, R2=ME, R3=H, R9] =isobutyl) (200 mg, 60%). [[0471] 1H] NMR (300 MHz, CD30D) 8 8.41 (d, [J=4.] 8, [1),] [8.] 28 (d, [J=9.] 6,1), 7.95 (s, 1), 5.35 (d, [J=5.] 4,1), 4.25 (m, 2), 3.99 (d, [J=10.] 8,1), 3.78 (d, [J=3.] 6,1), 3.55 [(DD,] [J=3.] 6,10. 8,1), 2.52 (m, 3), 2.15 (s, 3), 1.93 (m, 1), 1.02 (m, 12). MS (ES+): 413 (M+1). [[0472]] To compound lib (Rl=Me, [R=ME,] [R3=H,] R9 =isobutyl) (200 mg, 0.48 mmol) in water (10 mL), [ACOH] (2 [ML)] and MeOH (2 mL), [PTO2] (200 mg), was added, hydrogenated at 55 psi for 16 hours. After filtering the catalyst, the solvent was removed to obtain the crude material which on purification over silica gel column using 20 % MeOH in DCM as eluent. The lower Rf fraction provided the title compound (70 mg, 34%). [[0473] 1H] NMR (300 MHz, CD30D) 8 5.25 (d, J=5. 7,1), 4.20 [(DD,] J=9. 9; 3.3, 1), 4.07 (m, 2), 3.80 (d, J=3. 0,1), 3.60 (m, 2), 3.34 (m, 2), 2.84 (m, 1), 2.17 (m, 1), 2.10 (s, 3) 2.01 (m, 1), 1.77 (m, 3), 1.40 (m, 4), 0.91 (m, [12) ;] MS (ES+): 419 (M+1).
References:
WO2004/16632,2004,A2 Location in patent:Page 121-122
108-89-4
345 suppliers
$16.00/25mL
75-26-3
480 suppliers
$10.00/10g

4810-79-1
23 suppliers
$715.51/1G

108-89-4
345 suppliers
$16.00/25mL
187737-37-7
1 suppliers
inquiry

4810-79-1
23 suppliers
$715.51/1G
![1(4H)-Pyridinecarboxylic acid, 4-[bis(1-methylethoxy)phosphinyl]-4-(1-hydroxy-2-methylpropyl)-, ethyl ester](/CAS/20210305/GIF/882525-03-3.gif)
882525-03-3
0 suppliers
inquiry

4810-79-1
23 suppliers
$715.51/1G
![1,3-Dioxolane, 2-[2-(2,2-diethoxyethyl)-4-methylpentyl]-](/CAS/20210305/GIF/135351-44-9.gif)
135351-44-9
0 suppliers
inquiry

4810-79-1
23 suppliers
$715.51/1G