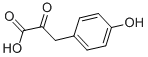
4-Hydroxyphenylpyruvic acid synthesis
- Product Name:4-Hydroxyphenylpyruvic acid
- CAS Number:156-39-8
- Molecular formula:C9H8O4
- Molecular Weight:180.16
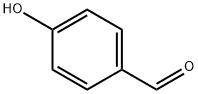
123-08-0
928 suppliers
$5.00/10g
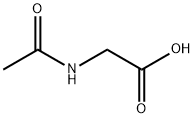
543-24-8
509 suppliers
$9.00/100g

156-39-8
186 suppliers
$27.00/250mg
Yield:156-39-8 25%
Reaction Conditions:
Stage #1:4-hydroxy-benzaldehyde;N-acetylglycine with sodium acetate;acetic anhydride at 80 - 100; for 5 h;
Stage #2: with water in acetone for 4 h;Reflux;
Stage #3: with hydrogenchloride;water for 6 h;Reflux;
Steps:
Method 1
General procedure: Step 1: In a 25-mL round-bottom flask equipped with a magnetic stir bar and a water condenser,glycine (5 g, 0.07 mol) and acetic anhydride (18.9 mL, 0.2 mol) were added into methanol (50mL) and the mixture was refluxed for 20 h. The N-acetylglycine product was purified byrecrystallization from methanol (white solid, 5.2 g, 67percent). Step 2: In a 25-mL round-bottom flask equipped with a magnetic stir bar and a water condenser,benzaldehyde or its derivative (5 mmol) was mixed with N-acetylglycine (702.6 mg, 6 mmol),sodium acetate (49.2 mg, 6 mmol) and acetic anhydride (3 mL). The resulting mixture wasstirred at 80 °C for 4 h, and then at 100 °C for 1 h. The resulting solution was stirred at roomtemperature for 12 h. The next day, 5 mL of ice-cold water was added. The precipitate wasisolated by vacuum filtration and rinsed with ~5 mL of cold water. This solid was then driedunder vacuum and used directly in the next step. Step 3: In a 50-mL round-bottom flask equipped with a magnetic stir bar and a water condenser,2 mmol of the product from Step 2 was dissolved in 10 mL of water/acetone (1:1). The resultingmixture was stirred under reflux for 4 h. The volatile solvent was removed by rotary evaporation,and the remaining residue was left in the refrigerator overnight to allow complete crystallizationof the product. The product was isolated by vacuum filtration and then transferred to 10 mL of 1M HCl. The mixture was stirred under reflux for 6 h. Finally, the solution was concentrated byrotary evaporation, and then the flask was placed inside a refrigerator overnight. The followingday, the crystals were collected by filtration, washed with ice-cold water, and dried undervacuum.
References:
Nguyen, Dat P.;Sladek, Rudolph N.;Do, Loi H. [Tetrahedron Letters,2020,vol. 61,# 32,art. no. 152196] Location in patent:supporting information
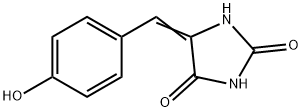
80171-33-1
22 suppliers
inquiry

156-39-8
186 suppliers
$27.00/250mg
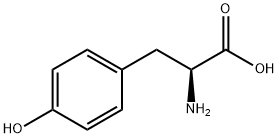
60-18-4
838 suppliers
$15.00/25g

156-39-8
186 suppliers
$27.00/250mg
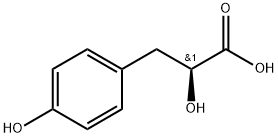
23508-35-2
74 suppliers
$90.00/50mg

60-18-4
838 suppliers
$15.00/25g

156-39-8
186 suppliers
$27.00/250mg
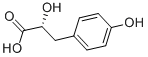
89919-57-3
37 suppliers
inquiry

60-18-4
838 suppliers
$15.00/25g

156-39-8
186 suppliers
$27.00/250mg