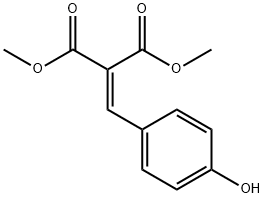
4-HYDROXY BENZYLIDENE MALONIC ACID DIMETHYL ESTER synthesis
- Product Name:4-HYDROXY BENZYLIDENE MALONIC ACID DIMETHYL ESTER
- CAS Number:51947-45-6
- Molecular formula:C12H12O5
- Molecular Weight:236.22
Yield:51947-45-6 99%
Reaction Conditions:
with piperidine;acetic acid in toluene at 130; for 20 h;Dean-Stark;Reflux;Knoevenagel Condensation;
Steps:
S2: Dimethyl 2-(4-hydroxybenzylidene)malonate
A 500 mL single neck round bottom flask with stirbar was charged with 4-hydroxybenzaldehyde (20.0 g, 163.8 mmol, 1.0 equiv), dimethyl malonate (18.8 mL, 163.8 mmol, 1.0 equiv), piperidine (1.62 mL, 16.4 mmol, 0.1 equiv), acetic acid (0.94 mL, 16.4 mmol, 0.1 equiv) and toluene (210 mL, 2.0 mol, 12.0 equiv) to give a brown suspension. The vessel was equipped with a reducing adapter and 14/20 Dean-Stark apparatus with 10 mL reservoir and reflux condenser. The heating mantle was set to 130 °C and the mixture stirred for 20 hours. The volume was reduced to approximately 50 mL by repeated emptying of the Dean-Stark trap. Upon cooling to room temperature, the dark brown solution solidified to a brown-yellow mass. The material was triturated with diethyl ether (50 mL). The mixture was vacuum filtered over an 8 cm Buchner funnel (Whatman qualitative filter paper size 1) leaving an orange filter cake. The orange filtrate was concentrated in vacuo and the residue triturated with diethyl ether (25 mL). A second crop was collected over a 3 cm Buchner funnel (Whatman qualitative filter paper size 1). The filtrate was discarded. The filter cakes were combined and dried in a vacuum oven to afford dimethyl 2-(4-hydroxybenzylidene)malonate (38 g, 99%) as an orange solid.
References:
Donahue, Matthew G.;Jentsch, Nicholas G.;Realini, Erin C. [Tetrahedron Letters,2017,vol. 58,# 33,p. 3219 - 3222] Location in patent:supporting information
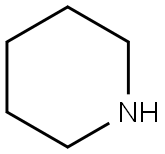
110-89-4
3 suppliers
$15.96/100ML
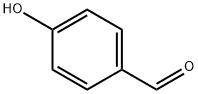
123-08-0
928 suppliers
$5.00/10g
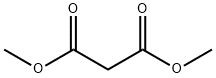
108-59-8
602 suppliers
$5.00/25g

51947-45-6
17 suppliers
inquiry