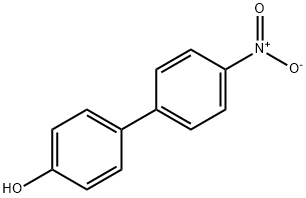
4-HYDROXY-4'-NITROBIPHENYL synthesis
- Product Name:4-HYDROXY-4'-NITROBIPHENYL
- CAS Number:3916-44-7
- Molecular formula:C12H9NO3
- Molecular Weight:215.2
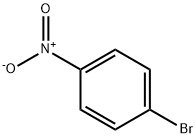
586-78-7
5 suppliers
$14.00/5g
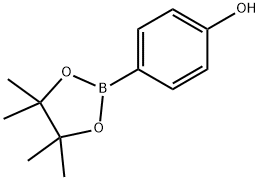
269409-70-3
254 suppliers
$11.00/1g

3916-44-7
140 suppliers
$16.00/1g
Yield:-
Reaction Conditions:
with tetrakis(triphenylphosphine) palladium(0);sodium carbonate in tetrahydrofuran;water;toluene at 80; for 5 h;Inert atmosphere;Suzuki Coupling;
Steps:
1 Synthesis of 4-(4-nitrophenyl)phenol
Under an inert gas atmosphere, 4- (4, 4, 6-trimethyl-1, 3, 2-dioxaborinan-2-yl) phenol (1.1 g, 5 mmol) 4-bromonitrobenzene (1.01 g, 5 mmol), tetrakistriphenylphosphine palladium (0.29 g, 0.25 mmol), 2 N aqueous sodium carbonate solution 4 ml, toluene 4 ml and THF Was stirred at 80 ° C. for 5 hours. After completion of the reaction, the mixture was filtered through celite with water and toluene, and the filtrate was washed three times with 40 ml of water. The organic layer was concentrated under reduced pressure. The precipitated crystals were washed with hexane and toluene to obtain 0.71 g of yellow brown crystals. As a result of GC analysis, the purity of the title compound was 95.4%. (Yield 62.7%). In place of 4- (4, 4, 6-trimethyl-1, 3, 2-dioxaborinan-2-yl) phenol, 4- (4, 4, 5, 5-tetramethyl-1, 3, 2-dioxaborolan-2-yl) phenol (CAS No. 269409-70-3) , The reaction was carried out under the same conditions as in Example 5 to obtain 0.6 g of crystals. As a result of GC analysis, the purity of the title compound was 88.0%. (Yield 48.8%)
References:
JP2016/222661,2016,A Location in patent:Paragraph 0068; 0069
939-49-1
0 suppliers
inquiry

3916-44-7
140 suppliers
$16.00/1g
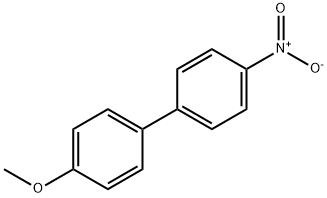
2143-90-0
69 suppliers
$31.00/100mg

3916-44-7
140 suppliers
$16.00/1g
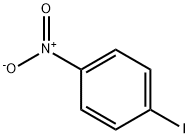
636-98-6
24 suppliers
$17.00/5g
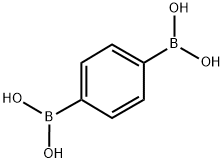
4612-26-4
298 suppliers
$6.00/1g

3916-44-7
140 suppliers
$16.00/1g
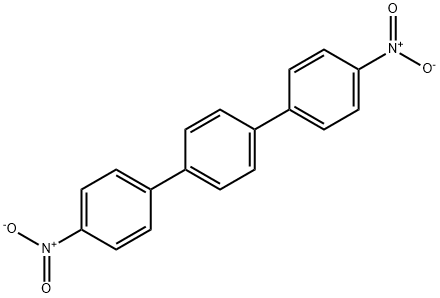
3282-11-9
49 suppliers
$19.00/250mg
937-31-5
182 suppliers
$8.00/250mg
54781-19-0
34 suppliers
$91.09/5ml

3916-44-7
140 suppliers
$16.00/1g