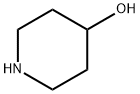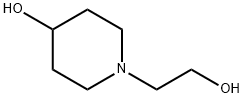
4-HYDROXY-1-PIPERIDINEETHANOL 96 synthesis
- Product Name:4-HYDROXY-1-PIPERIDINEETHANOL 96
- CAS Number:224431-84-9
- Molecular formula:C7H15NO2
- Molecular Weight:145.2
Yield:224431-84-9 60%
Reaction Conditions:
with sodium carbonate in ethanol; for 16 h;Heating / reflux;
Steps:
1
Piperidin-4-ol (5 g, 49.4 mmol) is dissolved in 200 ml of ethanol and anhydrous sodium carbonate (21 g, 197.6 mmol) is added. 2-Bromo-ethanol (6.9 ml, 98.8 mmol) is added dropwise and the reaction mixture is refluxed for 16 hours. After evaporation under reduced pressure the mixture is stirred with 200 mi of DCM and filtered. The clear filtrate is evaporated under reduced pressure und dried at high vacuum. Yield: 4.3 g (60%) of a colorless oil. MS (ESI) : 146 [M+H] +, 1 H-NMR (DMSO-d6) : 5 (ppm) 4.52 (d, 1H), 4.32 (t, 1H), 3.48 (dt, 2H), 3.4 (m, 1H), 2.7 (m, 2H), 2.35 (t, 2H), 2.05 (m, 2H), 1.68 (m, 2H), 1.3-1. 4 (m, 2H).
References:
WO2005/77932,2005,A2 Location in patent:Page/Page column 27; 37