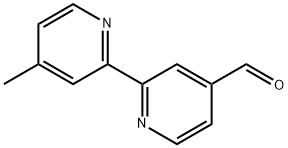
4-Formyl-4'-methyl-2,2'-bipyridine synthesis
- Product Name:4-Formyl-4'-methyl-2,2'-bipyridine
- CAS Number:104704-09-8
- Molecular formula:C12H10N2O
- Molecular Weight:198.22

141-78-6
630 suppliers
$20.50/250ml
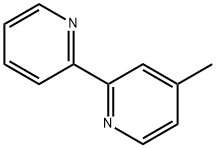
56100-19-7
68 suppliers
$82.00/250mg

104704-09-8
62 suppliers
inquiry
Yield: 49.1%
Reaction Conditions:
Stage #1:4-methyl-2,2'-bipyridine with selenium(IV) oxide in 1,4-dioxane for 24 h;Reflux;Inert atmosphere;
Stage #2:ethyl acetate for 1 h;Reflux;
Steps:
EXAMPLE. 4’-methyl-2,2’-bipyridine-4-carbaldehyde. The synthesis of 4’-methyl-2,2’-bipyridine-4-carbaldehydewas performed from a previously procedure (Peek et al., Int J Pept Protein Res, 38.2: 114-23 (1991)). In a 200 mLround-bottomed flask was 4’-methyl-2,2’-bipyridine (1.5 g, 8.14 mmol) in Dioxane (62.6 ml). Argon was bubble into thesolution for 15 minutes before the addition of selenium dioxide (1.012 g, 9.12 mmol). Argon was bubbled for another 20minutes before heating the solution at reflux for 24 hrs. After the flask was cooled to room temperature the solution wasfiltered and the solvent reduced. The remaining solid was dissolved in ethyl acetate and heated at reflux for 1 hr followedby a hot filtration. The filtrate was then washed with 0.1 M sodium carbonate and extracted with 0.3 M sodium metabisulfate.The pH of the aqueous layer was adjusted to 10 with sodium bicarbonate and the product was extracted withDCM. The solvent was removed under vacuum and no further purification was need for the white solid. (Yield: 49.1 %).
References:
Purdue Research Foundation;CHMIELEWSKI, Jean, A.;PIRES, Marcos, M.;PRZYBYLA, David, E. EP2300033, 2016, B1 Location in patent:Paragraph 0120
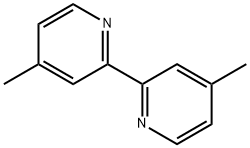
1134-35-6
291 suppliers
$5.00/1g

104704-09-8
62 suppliers
inquiry
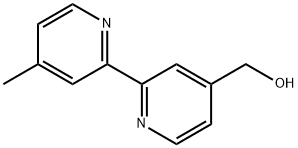
81998-04-1
55 suppliers
$30.00/100mg

104704-09-8
62 suppliers
inquiry

1134-35-6
291 suppliers
$5.00/1g

104704-09-8
62 suppliers
inquiry
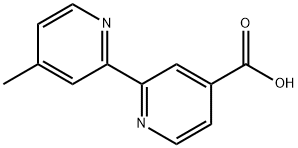
103946-54-9
87 suppliers
$80.00/50mg
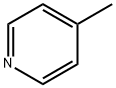
108-89-4
328 suppliers
$15.00/25mL

104704-09-8
62 suppliers
inquiry