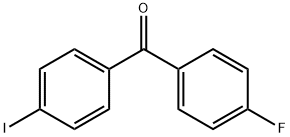
4-FLUORO-4'-IODOBENZOPHENONE synthesis
- Product Name:4-FLUORO-4'-IODOBENZOPHENONE
- CAS Number:141763-55-5
- Molecular formula:C13H8FIO
- Molecular Weight:326.1
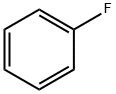
462-06-6
419 suppliers
$10.00/5g
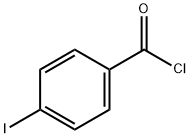
1711-02-0
161 suppliers
$10.00/1g

141763-55-5
11 suppliers
$126.00/1g
Yield:141763-55-5 83%
Reaction Conditions:
with aluminum (III) chloride at 40 - 50;
Steps:
1.1 1) Synthesis of intermediate [4-fluoro-4'-iodobenzophenone]
4-Iodobenzoyl chloride (5.00 g, 18.8 mmol) was added to 250 mLThe dry three-necked flask was charged with fluorobenzene (5.41 g, 56.3 mmol)With stirring, anhydrous aluminum chloride (3.88 g, 28.2 mmol)Warming to 40 ~ 50 , the reaction 5 ~ 6h. After the reaction,In a three-necked flask was dissolved in methylene chloride 50ml,Slowly add dilute hydrochloric acid, stirring until no precipitate.The reaction solution was then poured into a separatory funnel, extracted three times with dichloromethane,Then dilute hydrochloric acid washed 2 to 3 times until the water layer becomes colorless.The organic layer was dried over anhydrous sodium sulfate, filtered,The filtrate was rotary dried on a rotary evaporator to give 5.06 g of a yellow-white solid. Yield 83%.
References:
CN106565781,2017,A Location in patent:Paragraph 0048; 0049; 0050; 0051
![Benzene, 1-[difluoro(4-fluorophenyl)methyl]-4-iodo-](/CAS/20210305/GIF/142687-15-8.gif)