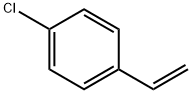
4-Chlorostyrene synthesis
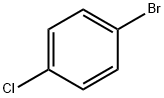
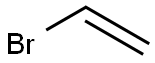
- Product Name:4-Chlorostyrene
- CAS Number:1073-67-2
- Molecular formula:C8H7Cl
- Molecular Weight:138.59
Yield:1073-67-2 82%
Reaction Conditions:
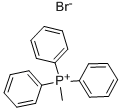
Stage #1:Methyltriphenylphosphonium bromide with 1,8-diazabicyclo[5.4.0]undec-7-ene in dichloromethane for 0.5 h;Reflux;
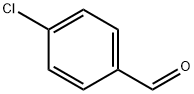
Stage #2:4-chlorobenzaldehyde in dichloromethane for 5 h;Reflux;
Steps:
Comparative example
In a 25mL round-bottom flask, add 2mmol (0.714g) of methyltriphenylphosphine bromide and 10mL of methylene chloride solution, and add DBU (an organic base, namely 1,8- Diazabicycloundec-7-ene) 2.2 mmol (0.339 g). After heating to reflux for 30 minutes,A 1 mmol (0.141 g) solution of p-chlorobenzaldehyde in dichloromethane (3 mL) was added to the round bottom flask. After that, it was heated to reflux for 5 hours. After the reaction was completed, water was added to the reaction system to perform an extraction operation, and the methylene chloride reaction system was washed with water three times (each time 5 mL of water was added). After collecting the methylene chloride solution in the lower layer of the separatory funnel, the dichloromethane was dried over anhydrous magnesium sulfate The methane solution was filtered to remove magnesium sulfate and spin-dried (dichloromethane) to obtain a crude product as a light yellow oil. The oil was subjected to silica gel column chromatography and eluted with n-hexane to obtain 0.82 mmol (0.114g) of p-chlorostyrene (Yield 82%), followed by elution with dichloromethane to obtain 0.91 mmol (0.253 g) of triphenylphosphine oxide.
References:
Tsinghua University;Wang Yin;Xu Chao;Chen Jing CN111056927, 2020, A Location in patent:Paragraph 0069-0070
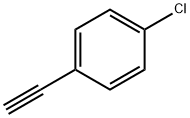
873-73-4
228 suppliers
$5.00/100mg

1073-67-2
311 suppliers
$7.00/5g
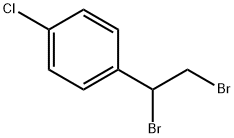
23135-16-2
24 suppliers
$70.00/2.5g

1073-67-2
311 suppliers
$7.00/5g
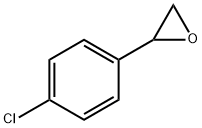
2788-86-5
72 suppliers
$40.00/100mg

1073-67-2
311 suppliers
$7.00/5g