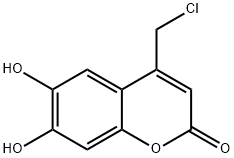
4-(chloromethyl)-6,7-dihydroxy-2-benzopyrone synthesis
- Product Name:4-(chloromethyl)-6,7-dihydroxy-2-benzopyrone
- CAS Number:85029-91-0
- Molecular formula:C10H7ClO4
- Molecular Weight:226.61
Yield: 61%
Reaction Conditions:
with sulfuric acid at 0; for 1 h;
Steps:
4-Chloromethyl-6,7-dihydroxyl-coumarin (compound C).
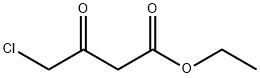
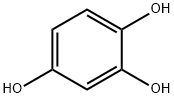
1,2,3-trihydroxybenzene (6.31 g, 50 mmoL) was dissolved in conc. H2SO4 (2 mL) and ethyl 4-chloroacetoacetate (8.23 g, 6.76 mL, 50 mmoL) was added to the solution. The reaction mixture was stirred at 0 °C for 1 h. Then ice and water (100 mL) were added, the suspension was filtered and washed with cold water. The obtained solid was recrystallized in ethanol and dried under vacuum overnight to give compound C as a yellow solid (6.91 g, 61%). mp 265-266 °C; FTIR (ATR, νmax, cm-1): 3272 (OH), 3094 (=C-H), 2971 (-C-H), 1738 (C=O), 1659 (lactone C=O), 1621 (C=C), 1582, 1389 (aromatic ring), 1227 (C-O), 865 (C-Cl); 1H NMR (500 MHz, DMSO-d6): δH 4.91 (2H, s, CH2Cl), 6.41 (1H, s, =CHC=O), 6.79 (1H, s, C8-H aromatic), 7.13 (1H, s, C5-H aromatic), 9.46 (1H, br s, C6-OH), 10.39 (1H, br s, C7-OH); 13C NMR (125 MHz, DMSO-d6): δC 41.6, 102.9, 108.9, 109.4, 111.2, 142.9, 148.3, 150.6, 150.7, 160.5; UV-Vis λmaxHEPES/MeOH (20/80) (ε M-1cm-1) 215 (1.63 x 104), 348 (1.11 x 104) nm; HMRS (ESI): m/z calcd. for C10H8ClO4 [M+H]+, 227.0111; found, 227.0100.
References:
Loarueng, Chutipapha;Boekfa, Bundet;Jarussophon, Suwatchai;Pongwan, Pawinee;Kaewchangwat, Narongpol;Suttisintong, Khomson;Jarussophon, Nongpanga [Arkivoc,2019,vol. 2019,# 6,p. 116 - 127]