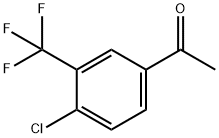
4'-CHLORO-3'-(TRIFLUOROMETHYL)ACETOPHENONE synthesis
- Product Name:4'-CHLORO-3'-(TRIFLUOROMETHYL)ACETOPHENONE
- CAS Number:129825-11-2
- Molecular formula:C9H6ClF3O
- Molecular Weight:222.59
![1-[4-CHLORO-3-(TRIFLUOROMETHYL)PHENYL]ETHAN-1-OL](/CAS/GIF/348-84-5.gif)
348-84-5
19 suppliers
inquiry

129825-11-2
131 suppliers
$14.00/5g
Yield: 95%
Reaction Conditions:
with oxalyl dichloride;triethylamine in dichloromethane;dimethyl sulfoxide at -78;
Steps:
22 Example 22 Preparation of 4-Chloro-3- (trifluoromethyl) phenacyl bromide
[4-CHLORO-3- (TRIFLUOROMETHYL)] benzylaldhyde (3.0 g, 14.4 mmol) in diethyl ether was cooled [TO-78 C. METHYLMAGNESIUM] chloride (3. 0M solution in THF, 29 mmol) was added dropwise. The reaction was warmed up slowly [TO-20] C. TLC showed the reaction was complete. It was quenched with saturated amonium chloride solution. The aqueous layer was extracted with ethyl acetate, organic layer wre combined and washed with water, brine, and dried with magnesium sulfate. This gave [1- (4-CHLORO-3-TRIFLUOROMETHYLPHENYL)] ethanol (3.2g, [99%)] after the solvent was removed under reduced pressure. [1- (4-CHLORO-4-TRIFLUOROMETHYL-] phenyl) ethanol was then oxidized by [SWERN] oxidation (2.5 eq. DMSO, 1.2 eq. oxyal chloride, 5 eq. triethylamine, [60ML] of [DICHLOROMETHANE,-78] C). The resulting 1- (3-chloro-4- trifluoromethylphenyl) ethanone (3 g, 95% yield) was brominated according to a literature [PROCEDURE (HORNG-CHIH, HUANG ET AL. , J. MED. CHEM. ; 1996, VOL. 39,253-266). 4-CHLORO-3- (TRIFLUOROMETHYL) -PHENACYL BROMIDE WAS OBTAINED IN 70% YIELD. LC/MS (ES+) M/E 303 [M+1]]
References:
SMITHKLINE BEECHAM CORPORATION WO2003/101970, 2003, A1 Location in patent:Page 10-11
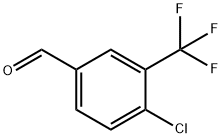
34328-46-6
181 suppliers
$8.00/1g

129825-11-2
131 suppliers
$14.00/5g