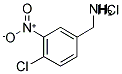
4-CHLORO-3-NITROBENZYLAMINE HYDROCHLORIDE synthesis
- Product Name:4-CHLORO-3-NITROBENZYLAMINE HYDROCHLORIDE
- CAS Number:116599-39-4
- Molecular formula:C7H8Cl2N2O2
- Molecular Weight:223.06
939-80-0
300 suppliers
$5.00/1g

116599-39-4
10 suppliers
inquiry
Yield:116599-39-4 65%
Reaction Conditions:
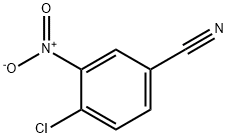
Stage #1: 4-chloro-3-nitrobenzonitrilewith borane-THF in tetrahydrofuran at 0 - 20;
Stage #2: with hydrogenchloride;methanol in tetrahydrofuran; for 4 h;Reflux;
Steps:
59.aa
Example 59N-(4-Bromo-phenyl)-2-(2-chloro-5-{[(1-trifluoromethyl-cyclopropanecarbonyl)-amino]- methyl}-phenylamino)-6-(2,2-difluoro-ethoxy)-1-methyl-1 /-/-benzimidazole-5-carboxylic acid amide(aa) (4-Chloro-3-nitrophenyl)methylamine hydrochlorideBH3/THF (279 ml 279 mmol) was added to a solution of 4-chloro-3-nitrobenzonitrile (30 g; 164 mmol) in THF (100 ml.) over 30 min at 0 0C and the resulting mixture was stirred over night and allowed to reach rt. MeOH (150 ml.) and cone. HCI (60 ml.) was added and the resulting mixture was refluxed for 4 h and thereafter concentrated. The residue was treated with water (500 ml.) and the resulting precipitate was filtered off. The filtrate was treated with NaCI (120 g) and heated and the resulting precipitate was filtered off to give the sub-title compound. Yield: 24.059 g (65%).
References:
WO2010/100249,2010,A1 Location in patent:Page/Page column 101-102