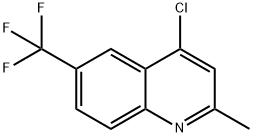
4-CHLORO-2-METHYL-6-TRIFLUOROMETHYLQUINOLINE synthesis
- Product Name:4-CHLORO-2-METHYL-6-TRIFLUOROMETHYLQUINOLINE
- CAS Number:867167-05-3
- Molecular formula:C11H7ClF3N
- Molecular Weight:245.63
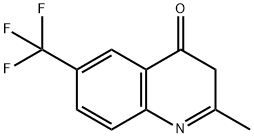
867167-03-1
2 suppliers
inquiry

867167-05-3
26 suppliers
$98.00/1g
Yield:-
Reaction Conditions:
with trichlorophosphate;N,N-dimethyl-formamide in tetrahydrofuran at 0 - 20; for 4 h;
Steps:
10.B
To 1.65 g (7.2 mmol) of the product from example 10, Step A in dry tetrahydrofuran (100 ml) containing a catalytic amount of dimethylformamide (0.5 ml) and pre-cooled to 0°C, was added dropwise 0.8 g (8.7 mmol) of phosphoiyl chloride. The reaction mixture was allowed to attain room temperature and stirred for 4 hours. It was diluted with water (20 ml) and the pH adjusted to 7 using saturated sodium bicarbonate solution. The reaction mixture was partitioned between water and ethyl acetate. The combined organic layers were successively washed with water (50 ml x 2) and brine (50 ml x 2), dried over anhydrous sodium sulphate and evaporated under vacuo to give 1.3 g of the title compound as red oil
References:
WO2005/97746,2005,A2 Location in patent:Page/Page column 119-120
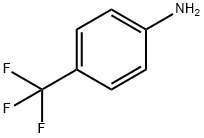
455-14-1
509 suppliers
$5.00/10g

867167-05-3
26 suppliers
$98.00/1g