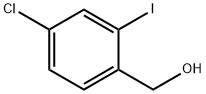
4-chloro-2-iodobenzyl alcohol synthesis
- Product Name:4-chloro-2-iodobenzyl alcohol
- CAS Number:244104-55-0
- Molecular formula:C7H6ClIO
- Molecular Weight:268.48
13421-13-1
178 suppliers
$7.00/1g

244104-55-0
21 suppliers
inquiry
Yield: 75%
Reaction Conditions:
Stage #1:4-chloro-2-iodobenzoic acid with chloroformic acid ethyl ester;triethylamine in tetrahydrofuran at 0; for 1.5 h;
Stage #2: with sodium tetrahydroborate in tetrahydrofuran for 2.5 h;
Steps:
6 Example 6: (4-Chloro-2-iodophenyl)methanol (8a)
4-chloro-2-iodobenzoic acid 7a (15 g, 53 mmol) was dissolved in dry THF (250 mL) and the solution was cooled to 0 °C. Hereupon, NEt3 (1 1 mL, 80 mmol) and ethyl chloroformate (7.6 mL, 80 mmol) were added. The reaction was stirred for 1.5 hour and subsequently NaBH (8.0 g, 210 mmol) was added in four portions. After 1.5 hour, additional NaBH (4.0 g, 105 mmol) was added and the reaction was stirred for another hour. Hereupon, the reaction was quenched with H20 (100 mL) and EtOAc (200 mL) was added. The organic layer was washed with H20 (3 χ 150 mL), brine (100 mL) and subsequently dried over MgS04. The solvents were removed under reduced pressure and the crude product was obtained by gradient column chromatography (EtOAc/-heptane, 1 :9 to 1 :6). Compound 8a was obtained as white solid (8.4 g, 75% over 2 steps). 1H-NMR (400 MHz, CDC13) δ: 7.82 (s, 1H), 7.45-7.33 (m, 2H), 4.65 (d, J = 6.2 Hz, 2H), 1.94 (t, J = 6.2 Hz, 1H). 13C- NMR (75 MHz, CDC13) δ: 141.1, 138.3, 133.8, 128.8, 128.6, 96.9, 68.6. HRMS (EI+) m/z calcd for C7H60C1I [M]'+ 267.9152, found 267.9160.
References:
STICHTING KATHOLIEKE UNIVERSITEIT;SYNAFFIX B.V.;DEBETS, Marjoke Froukje;RUTJES, Floris Petrus Johannes Theodorus;VAN HEST, Jan Cornelis Maria;VAN DELFT, Floris Louis;VAN BERKEL, Sander Sebastiaan WO2014/189370, 2014, A1 Location in patent:Page/Page column 29
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

244104-55-0
21 suppliers
inquiry
89-77-0
421 suppliers
$6.00/10g

244104-55-0
21 suppliers
inquiry