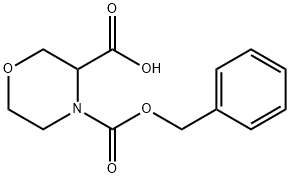
4-CBZ-MORPHOLINE-3-CARBOXYLIC ACID synthesis
- Product Name:4-CBZ-MORPHOLINE-3-CARBOXYLIC ACID
- CAS Number:256446-67-0
- Molecular formula:C13H15NO5
- Molecular Weight:265.26
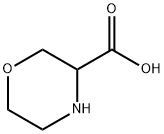
77873-76-8
118 suppliers
$21.00/100mg
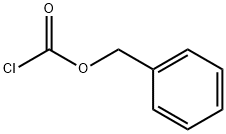
501-53-1
412 suppliers
$10.00/1g

256446-67-0
47 suppliers
$95.00/100mg
Yield:-
Reaction Conditions:
Stage #1: morpholine-3-carboxylic acid;benzyl chloroformatewith sodium hydroxide in water at 5 - 15; pH=6 - 9; for 2 h;
Stage #2: with hydrogenchloride in water; pH=0;
Steps:
1
Morpholine-3-carboxylic acid (10 g, 76 mmol) was dissolved in water (50 ml) and cooled to 5° C. A solution of 50% sodium hydroxide (6.10 g, 76 mmol) was added and the reaction mixture was cooled back to 5° C. An additional portion of 50% sodium hydroxide (7.93 g, 99 mmol) was then diluted to 22 mL with water and added to an addition funnel. Benzyl chloroformate (65.3 ml, 458 mmol) was added to a separate addition funnel and the two reagents were simultaneously added dropwise over about 30 minutes while maintaining the reaction temperature below 10° C. The pH of the mixture was about 6 when the addition was completed. Additional 50% NaOH (1.13 g, 14.3 mmol) was added to adjust pH to 9. The reaction was stirred at 10-15° C. for 2 hours. The reaction mixture was extracted with hexanes (2×30 ml) to remove excess benzyl chloroformate. EtOAc (50 ml) was added into the aqueous phase. The mixture was acidified to pH of about 0 with concentrated HCl (10 g). The organic phase was separated and the aqueous phase was extracted with EtOAc (3×100 ml). The combined organic solution was concentrated to afford an oil. The product was further dried under high vacuum overnight to afford 21.2 g of crude 4-(benzyloxycarbonyl)morpholine-3-carboxylic acid which was used in the next step without further purification. [M-H] calculated for C13H15NO5, 264. found, 264.
References:
US2011/53921,2011,A1 Location in patent:Page/Page column 14