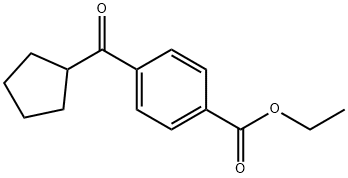
4-CARBOETHOXYPHENYL CYCLOPENTYL KETONE synthesis
- Product Name:4-CARBOETHOXYPHENYL CYCLOPENTYL KETONE
- CAS Number:898791-40-7
- Molecular formula:C15H18O3
- Molecular Weight:246.3
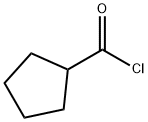
4524-93-0
136 suppliers
$13.43/1gm:
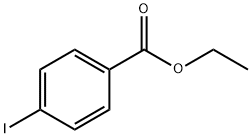
51934-41-9
289 suppliers
$6.00/5g

898791-40-7
14 suppliers
$417.00/1g
Yield:-
Reaction Conditions:
Stage #1: 4-iodobenzoic acid ethyl esterwith TurboGrignard in tetrahydrofuran at -40; for 0.833333 h;
Stage #2: copper(l) iodide in tetrahydrofuran at -15; for 0.133333 h;Grignard Reaction;
Stage #3: Cyclopentanecarboxylic acid chloride in tetrahydrofuran at -40 - 0; for 3 h;
Steps:
Intermediate (31): ethyl 4-(cyclopentanecarbonyl)benzoate At -40° C., isopropylmagnesium chloride lithium chloride (13.9 mL, 1.3 M in THF) was added dropwise to a solution of ethyl 4-iodobenzoate (4971 mg, 18.01 mmol) in tetrahydrofuran (30 mL). The solution was stirred at -40° C. for 50 minutes. Copper (I) iodide (1.03 g, 5.4 mmol) was added. The mixture was allowed to warm to -15° C. and stir for 8 minutes. The solution was cooled back to -40° C. and cyclopentanecarbonyl chloride (3580 mg, 27.0 mmol) was added dropwise. The mixture was allowed to gradually warm to 0° C. over 3 hours. The mixture was quenched with 1 N HCl (20 mL) and diluted with ethyl acetate. The mixture was stirred at room temperature for 5 min. A white precipitate formed. The mixture was filtered through celite and the filtrate transferred to a separatory funnel. The layers were separated. The aqueous was extracted twice with ethyl acetate. The combined organics were washed with brine, dried over sodium sulfate, filtered and concentrated. Purification by column chromatography (0-20% ethyl acetate in heptane) gave ethyl 4-(cyclopentanecarbonyl)benzoate as an oil. 1H NMR (400 MHz, CDCl3, δ): 8.13-8.08 (m, 2H), 8.02-7.97 (m, 2H), 4.39 (q, J=7.22 Hz, 2 H), 3.77-3.65 (m, 1H), 1.99-1.79 (m, 4H), 1.77-1.60 (m, 4H), 1.40 (t, J=7.22 Hz, 3H).
References:
US2012/202834,2012,A1 Location in patent:Page/Page column 23