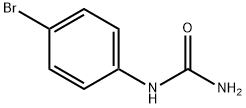
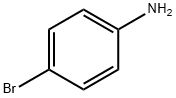
4-BROMOPHENYLUREA synthesis
- Product Name:4-BROMOPHENYLUREA
- CAS Number:1967-25-5
- Molecular formula:C7H7BrN2O
- Molecular Weight:215.05
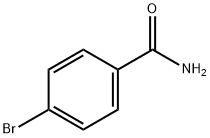
698-67-9
160 suppliers
$12.00/5g

1967-25-5
103 suppliers
$27.00/5g
Yield: 73%
Reaction Conditions:
with [bis(acetoxy)iodo]benzene;ammonia in methanol at 0 - 20; for 2 h;Inert atmosphere;Hofmann Rearrangement;
Steps:
N-Substituted Ureas; General Procedure A
General procedure: (Diacetoxyiodo)benzene (1.0 mmol, 2.0 equiv) was added in one portion to a stirred solution of the amide (0.5 mmol, 1.0 equiv) in NH3/MeOH (7 M, 1.25 mL, 17.5 equiv) at 0 °C under argon. After 30 min at 0 °C, the reaction mixture was allowed to reach room temperature and was left to stir for 90 min. After completion (monitored by TLC and 1H NMR), the reaction mixture was concentrated under reduced pressure and the crude product was purified by flash chromatography on silica gel.
References:
Franck, Xavier;Glachet, Thomas;Ibert, Quentin;Lohier, Jean-Fran?ois;Reboul, Vincent;Saraiva Rosa, Nathalie [Synthesis,2020,vol. 52,# 14,p. 2099 - 2105]
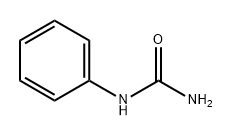
64-10-8
201 suppliers
$15.00/25g

1967-25-5
103 suppliers
$27.00/5g
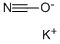
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)