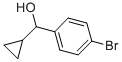
(4-BROMOPHENYL)(CYCLOPROPYL)METHANOL synthesis
- Product Name:(4-BROMOPHENYL)(CYCLOPROPYL)METHANOL
- CAS Number:70289-39-3
- Molecular formula:C10H11BrO
- Molecular Weight:227.1
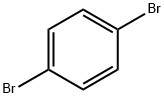
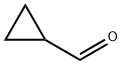
589-87-7
529 suppliers
$10.00/1g
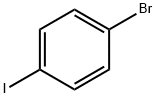
1489-69-6
316 suppliers
$6.00/1g
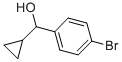
70289-39-3
27 suppliers
$106.00/1g
Yield:70289-39-3 71%
Reaction Conditions:
Stage #1: 1,4-bromoiodobenzenewith isopropyl magnesium chloride - lithium chloride complex in tetrahydrofuran at -20; for 0.666667 h;Inert atmosphere;
Stage #2: Cyclopropanecarboxaldehyde in tetrahydrofuran at 20; for 2 h;Inert atmosphere;
Steps:
1-((4-(cyclopropylmethyl)phenyl)sulfonyl)dispiro[pyrrolidine-3,1'-cyclobutane- 3',1''-[1,2]oxaborolo[4,3-d]pyrrolo[2,3-b]pyridin]-3''(6''H)-ol
The title compound was prepared by the scheme and procedures shown below: To a solution of 1-bromo-4-iodobenzene (2.8 g, 10 mmol) in THF (20 mL) was added iPrMgCl-LiCl (1.3N in THF, 10 mL) at -20oC under argon atmosphere. The reaction was stirred at -20oC for 40 min, and then cyclopropanecarbaldehyde (700 mg, 10.0 mmol) in THF (10 mL) was added. The reaction was allowed to warm to room temperature for 2h, quenched with water and extracted with EtOAc. The organic was concentrated, and the residue was purified by column chromatography to give (4-bromophenyl)(cyclopropyl)methanol (1.6 g, yield 71%) as a light yellow oil.1H NMR (300 MHz, CDCl3): δ 7.49 (d, J = 7.9 Hz, 2H), 7.40-7.20 (m, 3H), 3.98 (d, J = 8.3 Hz, 1H), 2.05 (br s, 1H), 1.21-1.08 (m, 1H), 0.75-0.53 (m, 2H), 0.53-0.28 (m, 2H) ppm. To a solution of (4-bromophenyl)(cyclopropyl)methanol (1.6 g, 7.1 mmol) in DCM (80 mL) was added TFA (1.2 g, 10.7 mmol) and Et3SiH (1.3 g, 10.7 mmol) at room temperature. The reaction was stirred for 30 min and concentrated. The residue was purified by column chromatography to give 1-bromo-4-(cyclopropylmethyl)benzene (1.3 g, yield 87%) as a light oil. 1H NMR (300 MHz, CDCl3): δ 7.42 (d, J = 7.9 Hz, 2H), 7.15 (d, J = 7.9 Hz, 2H), 2.51 (d, J = 6.8 Hz, 2H), 1.04 - 0.86 (m, 1H), 0.71 - 0.37 (m, 2H), 0.37 - 0.05 (m, 2H) ppm. To a solution of 1- bromo-4-(cyclopropyl-methyl)benzene (800 mg, 3.8 mmol) in THF (15 mL) was added n-BuLi (1.9 mL, 2.4 N) at -78oC under N2 atmosphere. The reaction was stirred at -78oC for 1 h, and then SO2 gas was bubbled into the reaction for 5 min. It was warmed to rt, and then DCM (10 mL) and NCS (612 mg, 4.6 mmol) were added. The reaction was stirred at rt for 2h, poured into water, extracted with DCM, and concentrated. The residue was purified by column chromatography to give 4-(cyclopropylmethyl)benzenesulfonyl chloride (600 mg, yield 69%) as a lightly yellow oil.1H NMR (300 MHz, CDCl3): δ 7.96 (d, J = 8.0 Hz, 2H), 7.51 (d, J = 8.1 Hz, 2H), 2.66 (d, J = 6.9 Hz, 2H), 1.00 (t, J = 7.5 Hz, 1H), 0.61 (d, J = 9.0 Hz, 2H), 0.25 (d, J = 6.0 Hz, 2H) ppm. To a solution of dispiro[pyrrolidine-3,1'-cyclobutane-3',1''-[1,2]oxaborolo[4,3- d]pyrrolo[2,3-b]pyridin]-3''(6''H)-ol (400 mg, crude) in THF (10 mL) were added Et3N (0.4 mL) and 4-(cyclopropylmethyl)-benzenesulfonyl chloride (230 mg, 1.0 mmol) at 0oC. The reaction mixture was stirred at room temperature for 30 min and concentrated. The residue was purified by pre-HPLC (TFA in ACN and H2O) to give the product 1-((4- (cyclopropylmethyl)phenyl)sulfonyl)-dispiro[pyrrolidine-3,1'-cyclobutane-3',1''-[1,2]oxaborolo[4,3- d]pyrrolo[2,3-b]pyridin]-3''(6''H)-ol (12.6 mg, yield 3%) as a white solid.1H NMR (400 MHz, DMSO-d6): δ 12.06 (s, 1H), 9.31 (br s, 1H), 8.47 (s, 1H), 7.77 (d, J = 8.2 Hz, 2H), 7.59 (s, 1H), 7.51 (d, J = 8.2 Hz, 2H), 6.66 (t, J = 1.7 Hz, 1H), 3.51 (s, 2H), 3.27 (t, J = 6.9 Hz, 2H), 2.67 (d, J = 13.7 Hz, 2H), 2.57 (d, J = 6.9 Hz, 2H), 2.04 (t, J = 6.9 Hz, 2H), 1.96 (d, J = 13.7 Hz, 2H), 0.95- 0.82 (m, 1H), 0.45 -0.26 (m, 2H), 0.13 (d, J = 4.8 Hz, 2H) ppm. HPLC purity: 98.55 % at 210 nm and 93.99% at 254 nm. MS (ESI+): m/z =464.2 (M+H)+.
References:
WO2021/61823,2021,A1 Location in patent:Paragraph 0176-0178
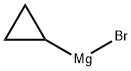
23719-80-4
176 suppliers
$85.20/25ml
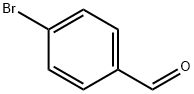
1122-91-4
690 suppliers
$9.00/10g
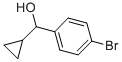
70289-39-3
27 suppliers
$106.00/1g
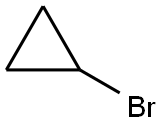
4333-56-6
384 suppliers
$6.00/5g

1122-91-4
690 suppliers
$9.00/10g
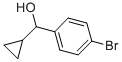
70289-39-3
27 suppliers
$106.00/1g
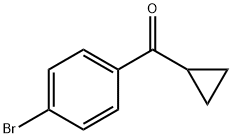
6952-89-2
133 suppliers
$19.00/1g
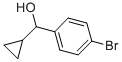
70289-39-3
27 suppliers
$106.00/1g