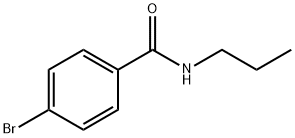
4-Bromo-N-propylbenzamide synthesis
- Product Name:4-Bromo-N-propylbenzamide
- CAS Number:223557-19-5
- Molecular formula:C10H12BrNO
- Molecular Weight:242.11
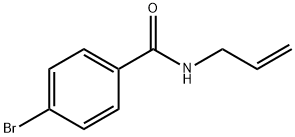
39887-27-9
9 suppliers
$31.00/250mg

223557-19-5
24 suppliers
$46.00/1g
Yield:223557-19-5 98%
Reaction Conditions:
with aluminum (III) chloride;manganese;cobalt(II) diacetate tetrahydrate;isopropyl alcohol at 22;Reagent/catalyst;
Steps:
Olefin Hydrogenation. General procedure with AlCl3.
General procedure: General procedure with AlCl3: This reaction may be carried out under ambient temperature and atmosphere. The olefin (0.2 mmol) (if solid), Mn powder (33 mg, 0.3 mmol), Co(OAc)2?4H2O (14.2 mg, 30 mol%) and AlCl3 (26.6 mg, 0.2 mmol) are weighed out and added to a 5 mL one-dram vial equipped with a stirring bar. A cap is placed on the reaction vessel and i-PrOH (2 mL) is added. The reaction is stirred vigorously for 4-20 h at rt. The reaction initially turns blue and over time turns black. The mixture is diluted with CH2Cl2, poured into a separating funnel and quenched with saturated aqueous NaHCO3 solution. After phase separation the aqueous phase is extracted twice further with CH2Cl2. The combined organic phases are washed with brine, dried over MgSO4 and filtered through a short plug of silica gel. The solvent is removed in vacuo to furnish the hydrogenated product without the need for further purification.
References:
van der Puyl, Vincent;McCourt, Ruairi O.;Shenvi, Ryan A. [Tetrahedron Letters,2021,vol. 72,art. no. 153047] Location in patent:supporting information
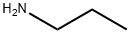
107-10-8
359 suppliers
$10.00/20mg
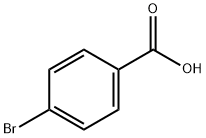
586-76-5
483 suppliers
$10.00/1g

223557-19-5
24 suppliers
$46.00/1g
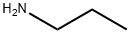
107-10-8
359 suppliers
$10.00/20mg
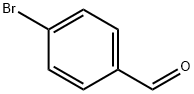
1122-91-4
685 suppliers
$9.00/10g

223557-19-5
24 suppliers
$46.00/1g
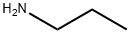
107-10-8
359 suppliers
$10.00/20mg
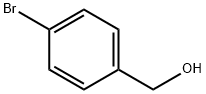
873-75-6
310 suppliers
$6.00/5g

223557-19-5
24 suppliers
$46.00/1g
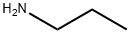
107-10-8
359 suppliers
$10.00/20mg
586-75-4
225 suppliers
$10.00/5g

223557-19-5
24 suppliers
$46.00/1g