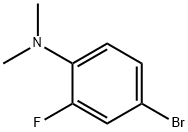
4-BROMO-N,N-DIMETHYL-2-FLUOROANILINE synthesis
- Product Name:4-BROMO-N,N-DIMETHYL-2-FLUOROANILINE
- CAS Number:887268-20-4
- Molecular formula:C8H9BrFN
- Molecular Weight:218.07

67-56-1
751 suppliers
$9.00/25ml
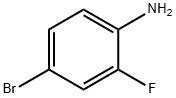
367-24-8
498 suppliers
$15.00/25g

887268-20-4
10 suppliers
inquiry
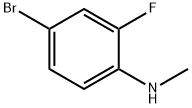
213190-12-6
14 suppliers
inquiry
Yield:213190-12-6 78% ,887268-20-4 4%
Reaction Conditions:
with hydrogenchloride;iron(III) oxide;titanium(IV) oxide in water; for 6 h;UV-irradiation;
Steps:
1
General procedure: A method for alkylation of aniline with Cu2O/TiO2 as a catalyst (loading amount is 0.01) and methanol as a carbon source, including the following processes:A 50 mL photoreaction flask with a stirrer was charged with 40 mL of methanol/water (water occupies 18% of the total volume of the reaction solvent (methanol+water)) solution, 250 mg of photocatalyst Cu2O/TiO, 5 mg of HCl and 0.2 mmol of substrate aniline.Then, the photoreaction flask was irradiated in a 365nm ultraviolet light source and stirred for 6 hours.Centrifuge the mixed solution system, add excess hydrochloric acid to adjust the pH to 1-2, and then rotate to evaporate all the methanol.The remaining aqueous reaction mixture was adjusted to pH 8 with sodium carbonate solution, and then extracted with water and ethyl acetate (5 times, 3 mL each time). The organic layer was washed with brine,Dry with Na2SO4 and evaporate to obtain a crude product.Distill and crystallize to obtain the product.The invention adopts a type II heterojunction photocatalyst, so that all the sub-steps of alkylation can be completed on the surface of the recombined catalyst under light conditions.After measurement, the final N-alkylation product yield was 99%, among which, the first-stage N-alkylation yield was 0.9%, and the second-stage N-alkylation yield was 99.1%. The results are shown in Table 1.
References:
CN112479894,2021,A Location in patent:Paragraph 0048-0056; 0058

367-24-8
498 suppliers
$15.00/25g
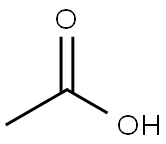
64-19-7
1559 suppliers
$10.00/25ML

887268-20-4
10 suppliers
inquiry
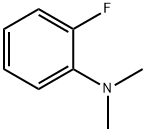
393-56-6
55 suppliers
$136.00/250mg

887268-20-4
10 suppliers
inquiry