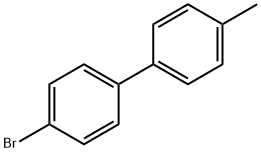
4-Bromo-4'-methylbiphenyl synthesis
- Product Name:4-Bromo-4'-methylbiphenyl
- CAS Number:50670-49-0
- Molecular formula:C13H11Br
- Molecular Weight:247.13
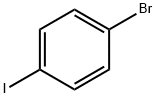
589-87-7
526 suppliers
$10.00/1g
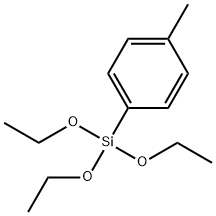
18412-57-2
44 suppliers
$38.00/1g

50670-49-0
125 suppliers
$22.00/250mg
Yield: 68%
Reaction Conditions:
with copper(l) iodide;2-benzhydryl-N,N-dimethylaniline;cesium fluoride in N,N-dimethyl-formamide at 120; for 48 h;Sealed tube;
Steps:
CuI-Catalyzed Couplings of Aryltriethoxysilanes with Aryl Iodidesand Bromides: General Procedure A (in the Presence of Ligand PN-1 or PN-2)
General procedure: In a glovebox, aryl iodide (1.0 mmol), CsF (228 mg, 1.5 mmol), CuI (19.0 mg, 0.10 mmol), and PN-1 ligand (30 mg, 0.10 mmol) were weighed into a 4-dram borosilicate scintillation vial and dissolved in DMF (5 mL). Aryltriethoxysilane (1.0 mmol) was then added to the mixture, and the vial was tightly capped with a poly-seal conelined urea cap (Wheaton). The mixture was taken out of the glove box and placed in an oil bath pre-heated to 120 °C with vigorous stirring. After 48 h, the mixture was cooled to r.t., diluted with EtOAc (15 mL) and washed with H2O (3 × 5 mL). The aqueous fraction was back-extracted with EtOAc (3 × 5 mL). The combined organic fractions were dried (Na2SO4) and cotton-filtered, and the solvent was removed on a rotary evaporator. The product was purified by column chromatography (silica gel, hexanes, hexanes-Et2O, or hexanes-EtOAc).
References:
Gurung, Santosh K.;Thapa, Surendra;Shrestha, Bijay;Giri, Ramesh [Synthesis,2014,vol. 46,# 14,art. no. SS-2014-C0146-ST,p. 1933 - 1937]

589-87-7
526 suppliers
$10.00/1g

5720-05-8
442 suppliers
$5.00/1g

50670-49-0
125 suppliers
$22.00/250mg
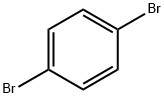
106-37-6
480 suppliers
$9.00/5g

5720-05-8
442 suppliers
$5.00/1g

50670-49-0
125 suppliers
$22.00/250mg
97295-31-3
15 suppliers
$500.95/5MG

589-87-7
526 suppliers
$10.00/1g

5720-05-8
442 suppliers
$5.00/1g

50670-49-0
125 suppliers
$22.00/250mg

97295-31-3
15 suppliers
$500.95/5MG
644-08-6
244 suppliers
$10.00/5g

50670-49-0
125 suppliers
$22.00/250mg