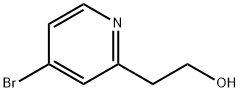
4-Bromo-(2-hydroxyethyl)-pyridine synthesis
- Product Name:4-Bromo-(2-hydroxyethyl)-pyridine
- CAS Number:98280-12-7
- Molecular formula:C7H8BrNO
- Molecular Weight:202.05

22282-99-1
391 suppliers
$6.00/5g

98280-12-7
32 suppliers
$65.00/10mg
Yield:-
Reaction Conditions:
Stage #1: 4-bromo-2-methylpyridinewith lithium diisopropyl amide in tetrahydrofuran;N,N-dimethyl-formamide at -78; for 1.25 h;
Stage #2: with sodium tetrahydroborate;acetic acid in methanol at -78 - 20; for 1 h;
Steps:
Intermediate I-94A: 2-(4-bromopyridin-2-yl)ethanol
4-bromo-2-methylpyridine (2.5 g, 14.53 mmol) was dissolved in anhydrous THF (50 mL) at -78 °C. LDA (25.4 mL, 50.9 mmol) was added to the reaction mixture which was allowed to stir at -78 °C for 30 minutes. Anhydrous DMF (3.38 mL, 43.6 mmol) wasthen added to the reaction mixture which continued to stir at -78 °C for an additional 45 minutes. A mixture of acetic acid (3.33 mL, 58.1 mmol)/MeOH (10 mL) was then added to the mixture at -78 °C followed by sodium borohydride (0.55 0 g, 14.53 mmol). The reaction mixture was then allowed to warm to room temperature and was stirred for 1 h then quenched with 10% aqueous citric acid solution at room temperature and stirred foran additional 10 minutes. The reaction mixture was extracted with EtOAc (3x). The organic layer was washed with brine (lx), dried with sodium sulfate, filtered and concentrated to yield Intermediate I-94A which was brought forward without further purification. LC-MS: Method H, MS (ESI) m/z: 201.9 (M+H). ‘H NMR (400MHz,DMSO-d6) 8.37 (d, J=5.3 Hz, 1H), 7.58 (d, J=1.8 Hz, 1H), 7.49 (dd, J5.4, 1.9 Hz, 1H), 3.78-3.70 (m, 1H), 2.89-2.86 (m, 2H), 2.28 (d, J=5.5 Hz, 1H).
References:
WO2018/13774,2018,A1 Location in patent:Page/Page column 226; 227

22282-99-1
391 suppliers
$6.00/5g
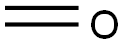
50-00-0
866 suppliers
$10.00/25g

98280-12-7
32 suppliers
$65.00/10mg

22282-99-1
391 suppliers
$6.00/5g
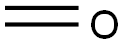
50-00-0
866 suppliers
$10.00/25g
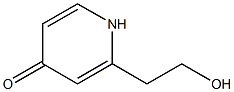
98547-36-5
2 suppliers
inquiry

98280-12-7
32 suppliers
$65.00/10mg