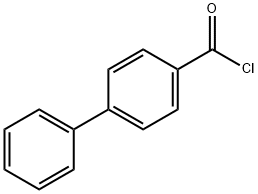
4-Biphenylcarbonyl chloride synthesis
- Product Name:4-Biphenylcarbonyl chloride
- CAS Number:14002-51-8
- Molecular formula:C13H9ClO
- Molecular Weight:216.66
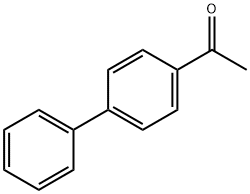
92-91-1
404 suppliers
$10.00/5g

14002-51-8
196 suppliers
$21.00/1g
Yield:14002-51-8 90%
Reaction Conditions:
Stage #1:biphenyl-4-acetaldehyde with pyridine;disulfur dichloride in chlorobenzene at 20; for 2 h;
Stage #2: with sulfuryl dichloride in chlorobenzene at 20 - 132; for 15.5 h;Reagent/catalyst;Temperature;
Steps:
11 Example 11: 4-Phenylbenzoyl chloride
To a mixture of 4-acetylbiphenyl (196 mg, 1.0 mmol), pyridine (0.012 ml, 0.15 mmol), and chlorobenzene (0.35 ml) was added S2CI2 (0.16 ml, 2.0 mmol) while stirring at room temperature. After 2 h SO2CI2 (0.121 ml, 1.5 mmol) was added dropwise, and the resulting mixture was stirred at room temperature for 0.5 h. The mixture was then stirred at 132 °C for 15 h. The mixture was diluted with CDCI3 (2 ml), an internal standard was added (1BU3PO4, 0.0552 ml, 0.20 mmol), and the mixture was analyzed. 1H NMR indicated that 4-phenylbenzoyl chloride had been formed in 90% yield. 1H NMR (PhCl, CDCI3, 400 MHz) delta = 8.06 (d, J = 8 Hz, 2H), 7.58 (d, J = 8 Hz, 2H), 7.53 (d, J = 8 Hz, 2H), 7.43-7.32 (m, 3H).
References:
LONZA LTD;ZARAGOZA DOERWALD, Florencio WO2017/5606, 2017, A1 Location in patent:Page/Page column 33-34
3218-36-8
337 suppliers
$6.00/5g

14002-51-8
196 suppliers
$21.00/1g
3597-91-9
241 suppliers
$27.00/5g

14002-51-8
196 suppliers
$21.00/1g
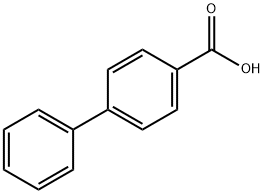
92-92-2
353 suppliers
$5.00/5g

14002-51-8
196 suppliers
$21.00/1g
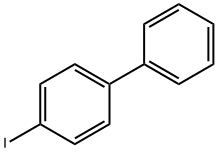
1591-31-7
336 suppliers
$8.00/5g
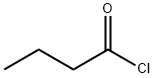
141-75-3
341 suppliers
$12.32/25G

14002-51-8
196 suppliers
$21.00/1g