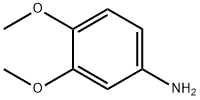
4-Aminoveratrole synthesis
- Product Name:4-Aminoveratrole
- CAS Number:6315-89-5
- Molecular formula:C8H11NO2
- Molecular Weight:153.18
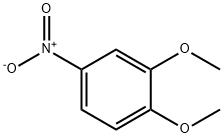
709-09-1
166 suppliers
$33.00/25g

6315-89-5
311 suppliers
$7.00/10g
Yield:6315-89-5 93%
Reaction Conditions:
with hydrazine;palladium on activated charcoal in ethanol;water for 24 h;Heating / reflux;
Steps:
3 Synthesis of 4-amino-1,2-dimethoxybenzene (9)
4-Nitro-1,2-dimethoxybenzene (8) (1.03 g, 5.62 mmol) was dissolved in 95 % (v/v) ethanol (60 ml) and hydrogenated with hydrazine hydrate (30 ml) over palladium on charcoal (0.11 g). The reaction was refluxed for 24 hours. The hot solution was filtered through celite and solvent removed under reduced pressure. Water was added and the product was extracted into dichloromethane, dried over sodium sulfate, filtered and solvent removed under reduced pressure to give a viscous liquid, which was then placed on ice to give 0.80 g of (9) as a white powder (5.22 mmol, 93 % yield). Melting point: 82-85 C (Literature mp: 83-87 C). 30 Decomposition at: 205 C. 1H n. m. r. (300 MHz, CDCl3): δ 6.66 (d, 1 H, J 8.4 Hz, Ar-H); 6.26 (d, 1 H, J 2.6 Hz, Ar-H); 6.18 (dd, 1 H, J 8.4 Hz, 2.6 Hz, Ar-H); 3.77 (s, 3H, [C1]-OCH3), 3.76 (s, 3H, [C2]-OCH3), 3.38 (br s, 2H, NH2). 13C n. m. r. (75 MHz, CDCl3): δ 148.9, (C1); 141.1, (C2); 139.8, (C4); 112.3, (C6); 105.5, (C5); 99.8, (C3); 55.7, ( [C1]-OCH3); 54.7, ([C2]-OCH3). IR (Nujol): 3380, br s; 1596, m; 1514, s; 11463, s; 1236, s; 849, m. Mass spectrum (ESI, +ve): m/z 153.8 [M]+.
References:
Monash University WO2003/99762, 2003, A1 Location in patent:Page 24-25
10535-17-8
80 suppliers
$41.00/100mg

6315-89-5
311 suppliers
$7.00/10g
2859-78-1
320 suppliers
$6.00/5g

6315-89-5
311 suppliers
$7.00/10g
2033-89-8
255 suppliers
$15.00/1g

6315-89-5
311 suppliers
$7.00/10g
91-16-7
563 suppliers
$13.00/25g

6315-89-5
311 suppliers
$7.00/10g