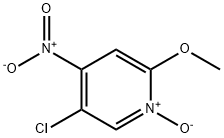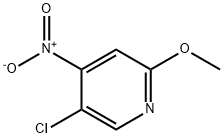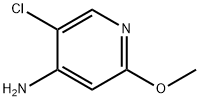
4-Amino-5-chloro-2-methoxypyridine synthesis
- Product Name:4-Amino-5-chloro-2-methoxypyridine
- CAS Number:719305-30-3
- Molecular formula:C6H7ClN2O
- Molecular Weight:158.59
719305-31-4
3 suppliers
inquiry

719305-30-3
12 suppliers
inquiry
Yield:719305-30-3 1.4 g
Reaction Conditions:
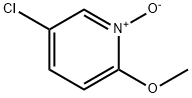
Stage #1: 5-chloro-2-methoxypyridine-1-oxidewith sulfuric acid;nitric acid at 20 - 70; for 20 h;
Stage #2: with iron;acetic acid at 80; for 1 h;
Steps:
1.2 Step 3: Synthesis of 5-chloro-2-methoxypyridin-4-amine
To a stirred solution of compound 5-chloro-2-methoxypyridine 1 - oxide (3g) in H2SCU (10.8ml_) was slowly added HNO3 (8.2ml_) at RT then heated to 70°C for 20h. TLC analysis indicated formation of two less polar spots. The reaction mixture was quenched in Ice water then basified with Na2C03 and extracted with EtOAc (6x100ml_). The combined organic layer was dried over Na2S04 and concentrated under reduced pressure to give a crude compound mixture (1.2g) as light yellow semi-solid which was used without further purificationTo a stirred solution of the mixture of 5-chloro-2-methoxy-4- nitropyridine 1 -oxide and 5-chloro-2-methoxy-4-nitropyridine (3.3g, 16.17mmol, 1 eq) in AcOH (40ml_) was added Fe (5.4g, 97.05mmol, 6eq) at RT and then heated to 80°C for 1 h. TLC analysis indicated formation of polar spot. The reaction mixture was quenched in Ice and basified with Na2C03 then filtered through celite bed, which is washed with EtOAc (4x50ml_). The filtrate was extracted with EtOAc (3x100ml_). The combined organic layer was dried over Na2S04 and concentrated under reduced pressure to give a crude product. The crude product was washed and triturated with n-pentane (2X10ml) to give the title compound (1 4g) as a light green solid; LCMS [M+H]+ 159.
References:
WO2019/153080,2019,A1 Location in patent:Paragraph 00374; 00375
13473-01-3
200 suppliers
$6.00/1g

719305-30-3
12 suppliers
inquiry