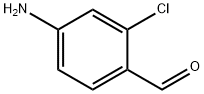
4-Amino-2-chlorobenzaldehyde synthesis
- Product Name:4-Amino-2-chlorobenzaldehyde
- CAS Number:42460-61-7
- Molecular formula:C7H6ClNO
- Molecular Weight:155.58
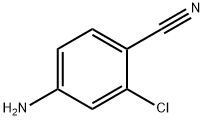
20925-27-3
166 suppliers
$10.00/5g

42460-61-7
17 suppliers
$65.00/10mg
Yield: 48%
Reaction Conditions:
Stage #1:4-amino-2-chlorobenzonitrile with diisobutylaluminium hydride in tetrahydrofuran at 20; for 2 h;Inert atmosphere;
Stage #2: with methanol at -10 - 20; for 2 h;
Steps:
63
Put 4-amino-2-chlorobenzonitrile (1.06 g, 6.947 mmol) into a three-necked flask, and replace the gas in the flask with a nitrogen ball three times to obtain a nitrogen-protected atmosphere. Anhydrous THF (11.58 mL) was injected with a syringe, and DIBAL-H (1.5M, 11.58 mL) solution was slowly added dropwise. After the dripping is finished,Stir at room temperature for 2 hours. After TLC detects that the reaction of the raw materials is complete, the temperature of the system is reduced to -10°C, methanol (2.08 mL) is slowly added dropwise, and the mixture is stirred at room temperature for 2 hours. Saturated potassium sodium tartrate solution was added dropwise, stirred at 45°C for 10 min, extracted with tert-butyl methyl ether three times, and the combined organic phase was washed with saturated aqueous sodium chloride solution and dried with anhydrous magnesium sulfate. Dry sample loading, column chromatography purification (petroleum ether/ethyl acetate=3:1) to obtain compound B16 as a yellow solid (514 mg, yield 48%)
References:
CN112608320, 2021, A Location in patent:Paragraph 0384-0395; 0396; 0840-0845
51420-25-8
25 suppliers
$174.16/1g

42460-61-7
17 suppliers
$65.00/10mg
121-86-8
204 suppliers
$14.00/5g

42460-61-7
17 suppliers
$65.00/10mg

121-86-8
204 suppliers
$14.00/5g

42460-61-7
17 suppliers
$65.00/10mg
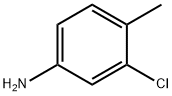
95-74-9
239 suppliers
$9.00/25g