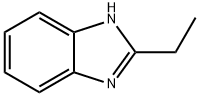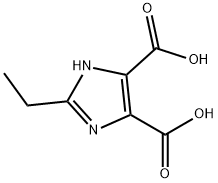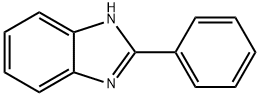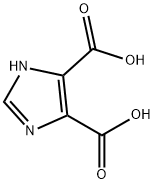
4,5-Imidazoledicarboxylic acid synthesis
- Product Name:4,5-Imidazoledicarboxylic acid
- CAS Number:570-22-9
- Molecular formula:C5H4N2O4
- Molecular Weight:156.1
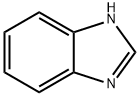
51-17-2
575 suppliers
$14.00/25g

570-22-9
297 suppliers
$10.00/5g
Yield:570-22-9 73%
Reaction Conditions:
with Iron(III) nitrate nonahydrate;tetrabutylammomium bromide;nitric acid in water at 80; for 8 h;
Steps:
18.1-18.3; 19.1-19.3; 22.1-22.3; 23.1-23.3; 24.1-24.3 Example 19
(1) Put 30 g of water into the reaction kettle, turn on the stirring, and sequentially add 0.06 g of Fe (NO3) 3.9H2O, 15 g of 96% sulfuric acid, 0.03 g of tetrabutylamine bromide, 2.5 g of benzimidazole, and raise the temperature;(2) When the reaction temperature reaches 80 ° C, 20 g of oxone is added in batches, and the reaction is incubated for 8 hours after the addition.(3) After the reaction is completed, the temperature is lowered to -10 to 10 ° C, and concentrated ammonia is slowly added dropwise under stirring to adjust the pH value to 0.6 to 1, and then stirring is continued for 1 hour for crystallization, suction filtration, and drying to obtain 4,5-imidazole di Carboxylic acid, the filtrate can be used for further product recovery, yield 73%.
References:
Changshu Institute of Technology;Yang Yang;Fu Renzhong;Wang Xin;Fang Zhengjiao;Lu Zhengyi;Zeng Xiaojun CN110818628, 2020, A Location in patent:Paragraph 0133-0140; 0149-0160
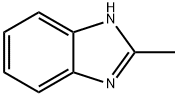
615-15-6
323 suppliers
$9.00/1g

570-22-9
297 suppliers
$10.00/5g
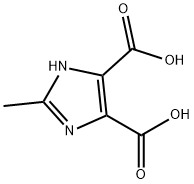
5313-35-9
31 suppliers
$109.00/50mg
1122-28-7
413 suppliers
$6.00/5g

570-22-9
297 suppliers
$10.00/5g