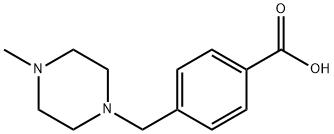
4-(4-Methylpiperazin-1-ylmethyl)benzoic acid synthesis
- Product Name:4-(4-Methylpiperazin-1-ylmethyl)benzoic acid
- CAS Number:106261-48-7
- Molecular formula:C13H18N2O2
- Molecular Weight:234.29
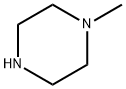
109-01-3
661 suppliers
$5.00/5 g
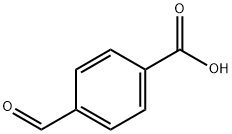
619-66-9
423 suppliers
$5.00/5g

106261-48-7
190 suppliers
$17.67/5gm:
Yield:106261-48-7 90%
Reaction Conditions:
with sodium tetrahydroborate;acetic acid in chloroform at 0 - 20; for 13 h;
Steps:
General procedure for the benzylation of 1-methylpiperazine (1). Synthesis of methyl 4-[(4-methylpiperazin-1-yl)methyl]benzoate (4a):
General procedure: AcOH (100%) (140 mL, 2.44 ml) was added over 1 h to a flask containing stirred NaBH4 (20.0 g, 0.53 ml) and CHCl3 (220 mL) at 0-5 °. The resulting mixture was stirred at 0-5 ° for 1.5 h and 1-methylpiperazine (1) (28.0 ml, 0.25 ml) and a solution of methyl 4-formylbenzoate (2a) (43.4 g, 0.26 ml) in CHCl3 (60 mL) were added. The resulting mixture was stirred at 0-5 ° for 1 h and then for 12 h at rt. the mixture was treated with H2O (150 mL) and Na2CO3 until pH 8.0-9.0. The aqueous phase was extracted with EtOAc (2 × 100 ml) then both organic layers were combined, washed with H2O (1 × 100 ml), and dried over anhydrous Na2SO4. Filtration and evaporation of the solvents gave methyl 4-[(4-methylpiperazin-1-yl)methyl]benzoate (4a): yellowish oil; yield: 61.6 g, 99%.
References:
Koroleva, Elena V.;Kadutskii, Aleksey P.;Farina, Alexander V.;Ignatovich, Janna V.;Ermolinskaya, Anastasiya L.;Gusak, Klaudiya N.;Kalinichenko, Elena N. [Tetrahedron Letters,2012,vol. 53,# 38,p. 5056 - 5058] Location in patent:experimental part
![METHYL 4-[(4-METHYLPIPERAZIN-1-YL)METHYL]BENZOATE](/CAS/GIF/314268-40-1.gif)
314268-40-1
82 suppliers
$90.00/500mg

106261-48-7
190 suppliers
$17.67/5gm:

109-01-3
661 suppliers
$5.00/5 g
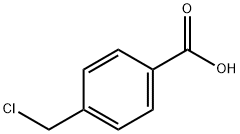
1642-81-5
385 suppliers
$17.00/5g

106261-48-7
190 suppliers
$17.67/5gm:
728936-43-4
1 suppliers
inquiry

106261-48-7
190 suppliers
$17.67/5gm:

109-01-3
661 suppliers
$5.00/5 g

106261-48-7
190 suppliers
$17.67/5gm: