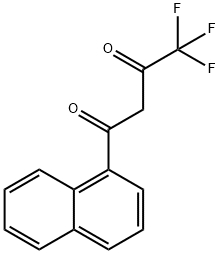
4,4,4-TRIFLUORO-1-(1-NAPHTHYL)BUTANE-1,3-DIONE synthesis
- Product Name:4,4,4-TRIFLUORO-1-(1-NAPHTHYL)BUTANE-1,3-DIONE
- CAS Number:7639-68-1
- Molecular formula:C14H9F3O2
- Molecular Weight:266.22
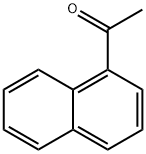
941-98-0
381 suppliers
$5.00/10g
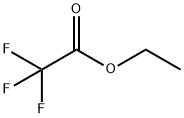
383-63-1
502 suppliers
$10.00/25g

7639-68-1
24 suppliers
$85.00/100mg
Yield:7639-68-1 76%
Reaction Conditions:
with methanol;sodium hydride in tetrahydrofuran;
Steps:
2.2.1. 4,4,4-Trifllluoro-1-(naphthalen-1-yl)butane-1,3-dione( 1-NaphH)
Sodium hydride (60% dispersion in mineral oil, 8.0 g, 200 mmol, 4eqv.) was placed in a three-necked round bottom flask equipped with amagnetic stirring bar, a refllux condenser, a dropping funnel and anargon inlet, and washed with three 50 mL portions of dry hexane toremove the mineral oil. This operation was conducted under argonblanket. Then 100 mL of THF was added and the suspension was stirredfor 5 min. A solution of 1-acetylnaphthalene (8.52 g, 50 mmol) and ethyltriflluoroacetate (9.2 g, 7.4 mL, 66 mmol) in 40 mL of dry THF wasprepared separately. Approximately 1/15 by volume of this solution wasadded to the NaH suspension in one portion, followed by addition of 0.5mL of dry MeOH as the catalyst. The rest of the solution was then addeddropwise to maintain a moderate evolution of hydrogen. The resultingdark mixture was stirred for 3 h and left overnight. Next day, 10 mL ofdry MeOH was added to the reaction mixture with external cooling by anice-water bath, followed by addition of 11.5 mL (12 g, 200 mmol) ofglacial AcOH. The mixture was evaporated to dryness under diminishedpressure, 100 mL of 5% aqueous HCl solution was added, and thediketone was extracted three times with 50 mL portions of EtOAc. Theorganic phase was washed with water until a neutral pH was obtained,then with 50 mL of brin, and then washed over MgSO4. Evaporation ofthe solvent gave a solid yellow product. An analytical sample wasrecrystallized from heptane. M.p. 43-45 C (lit. 45-48 C [44].Light-yellow powder.Yield - 10.15 g (76%).For C14H9F3O2 (FW 266.22)Calcd. C, 63.16; H, 3.41;Found C, 63.29; H, 3.37.1H NMR (600 MHz, CD2Cl2), (ppm): 15.22 (br.s., 1H), 8.53 (d, 1H,3JH H = 8.3 Hz), 8.12 (d, 1H, 3JH H = 8.2 Hz), 7.99 (d, 1H, 3JH H = 8.0Hz), 7.91 (dd, 1H, 3JH H = 6.9 Hz, 4JH H = 1.4 Hz), 7.68 (ddd, 1H,3JH H = 8.3 Hz, 3JH H = 6.8 Hz, 4JH H = 1.4 Hz), 7.64 (td, 1H, 3JH H =7.7 Hz, 3JH H = 8.0 Hz), 7.61 (ddd, 1H, 3JH H = 6.9 Hz, 4JH H = 8.2 Hz),6.60 (s, 1H);13C NMR (151 MHz, CD2Cl2), : 191.5, 174.3 (q, 2JC F = 36.6 Hz),133.9, 133.7, 132.0, 129.9, 128.8, 128.7, 128.0, 126.8, 125.1, 124.7,118.4 (q, 1JC F = 282.5 Hz), 97.6 (q, 3JC F = 2.2 Hz).19F NMR(282 MHz, CD2Cl2), : 76.53 (s, 3F);
References:
Metlin, Mikhail T.;Goryachii, Dmitry O.;Aminev, Denis F.;Datskevich, Nikolay P.;Korshunov, Vladislav M.;Metlina, Daria A.;Pavlov, Alexander A.;Mikhalchenko, Lyudmila V.;Kiskin, Mikhail A.;Garaeva, Veronika V.;Taydakov, Ilya V. [Dyes and Pigments,2021,vol. 195,art. no. 109701]