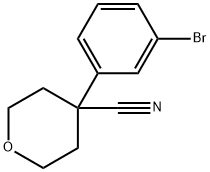
4-(3-BROMOPHENYL)TETRAHYDROPYRAN-4-CARBONITRILE synthesis
- Product Name:4-(3-BROMOPHENYL)TETRAHYDROPYRAN-4-CARBONITRILE
- CAS Number:245439-36-5
- Molecular formula:C12H12BrNO
- Molecular Weight:266.13

5414-19-7
194 suppliers
$6.00/5g
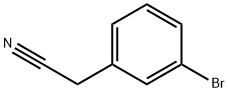
31938-07-5
238 suppliers
$8.00/5g

245439-36-5
25 suppliers
$348.00/1g
Yield:245439-36-5 93%
Reaction Conditions:
Stage #1: (3-bromophenyl)acetonitrilewith sodium hydride in N,N-dimethyl-formamide at 0; for 0.25 h;
Stage #2: 1,1'-oxybis(2-bromo-ethane) in N,N-dimethyl-formamide at 0 - 20; for 4 h;
Steps:
Intermediate Q104-(3 -Bromophenyl)tetrahydro-2H-pyran-4-carbonitrile
NaH (306 mg, 7.65 mmol) was washed with hexanes, DMF was added and the mixture was cooled to 0 °C. A solution of 2-(3-bromophenyl)acetonitrile (0.5 g, 2.55 mmol) in DMF was added dropwise using an addition funnel. After stirring for 15 mm at 0 °C, a solution of 1-bromo-2-(2-bromoethoxy)ethane (0.62 g, 2.68 mmol) in DMF was added slowly. The reaction mixture was allowed to warm up to room temperature andstirred for 4 h, NH4C1 solution was added carefully. The mixture was extracted (3 X) with EA, the organic layer was isolated, dried and concentrated to give 4-(3- bromophenyl)tetrahydro-2H-pyran-4-carbonitrile (0.63 g, 93% yield) white solid. ‘H NMR (400MHz, chloroform-d) ? 7.65 (t, J=1.9 Hz, 1H), 7.52 (ddd, J=7.9, 1.9, 1.0 Hz,1H), 7.46 (ddd, J=8.0, 1.9, 0.9 Hz, 1H), 7.33 (t, J=7.9 Hz, 1H), 4.16-4.07 (m, 2H), 3.92 (td, J=12.0, 2.4 Hz, 2H), 2.24-1.98 (m, 4H).
References:
WO2016/64957,2016,A1 Location in patent:Page/Page column 160; 161

31938-07-5
238 suppliers
$8.00/5g
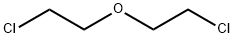
111-44-4
330 suppliers
$13.00/50g

245439-36-5
25 suppliers
$348.00/1g
108-20-3
562 suppliers
$25.00/25mL

31938-07-5
238 suppliers
$8.00/5g

111-44-4
330 suppliers
$13.00/50g

245439-36-5
25 suppliers
$348.00/1g

31938-07-5
238 suppliers
$8.00/5g
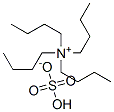
32503-27-8
629 suppliers
$5.00/25g

111-44-4
330 suppliers
$13.00/50g

245439-36-5
25 suppliers
$348.00/1g