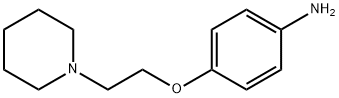
4-(2-piperidinoethoxy)aniline synthesis
- Product Name:4-(2-piperidinoethoxy)aniline
- CAS Number:38948-27-5
- Molecular formula:C13H20N2O
- Molecular Weight:220.31
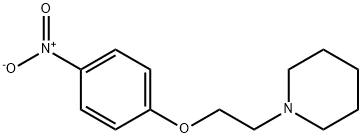
92033-76-6
10 suppliers
$131.00/5g

38948-27-5
30 suppliers
$65.00/100mg
Yield:38948-27-5 100%
Reaction Conditions:
with hydrogen;palladium 10% on activated carbon in ethanol; under 2068.65 Torr; for 0.5 h;
Steps:
14.C
C. 4-(2-Piperidin-1-yl-ethoxy)-phenylamine. To a solution of 1-[2-(4-nitro-phenoxy)-ethyl]-piperidine (300 mg, 1.20 mmol) in EtOH (50 mL) was added 10% Pd/C (50 mg). The mixture was placed on a Parr hydrogenator at 40 psi of H2 for 30 min. The resultant mixture was filtered through diatomaceous earth, and the filtrate was concentrated to give a clear and colorless oil (264 mg, 100%). 1H NMR (400 MHz, DMSO-d6): 6.64 (d, J=8.7, 2H), 6.50 (d, J=8.0, 2H), 4.58 (s, 2H), 3.90 (t, J=6.0, 2H), 2.57 (t, J=6.0, 2H), 2.39 (s, 4H), 1.52-1.44 (m, 4H), 1.41-1.34 (m, 2H).
References:
US2006/223792,2006,A1 Location in patent:Page/Page column 27
![Carbamic acid, N-[4-[2-(1-piperidinyl)ethoxy]phenyl]-, 1,1-dimethylethyl ester](/CAS/20210305/GIF/866021-30-9.gif)
866021-30-9
0 suppliers
inquiry

38948-27-5
30 suppliers
$65.00/100mg
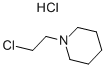
2008-75-5
263 suppliers
$10.00/1g
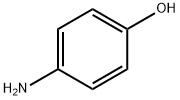
123-30-8
616 suppliers
$10.00/5g

38948-27-5
30 suppliers
$65.00/100mg
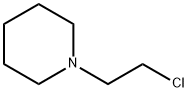
1932-03-2
96 suppliers
$50.00/100mg
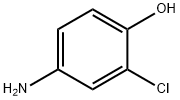
3964-52-1
249 suppliers
$20.00/5g

38948-27-5
30 suppliers
$65.00/100mg
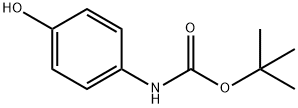
54840-15-2
108 suppliers
$8.00/1g

38948-27-5
30 suppliers
$65.00/100mg