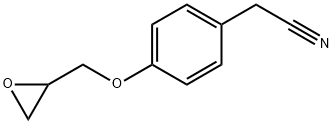
4-(2-OxiranylMethoxy)benzeneacetonitrile synthesis
- Product Name:4-(2-OxiranylMethoxy)benzeneacetonitrile
- CAS Number:35198-42-6
- Molecular formula:C11H11NO2
- Molecular Weight:189.21
Yield:35198-42-6 82%
Reaction Conditions:
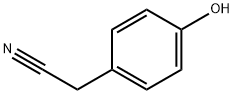
Stage #1: 4-cyanomethylphenol;epichlorohydrinwith tetrabutylammomium bromide at 68; for 6 h;
Stage #2: with water;sodium hydroxide in acetone; for 3 h;
Steps:
2.2 (2) Preparation of 4- (2-oxiranemethoxy) phenylacetonitrile
Add p-hydroxyphenylethyl cyanide (0.01 mol, 1.33 g) and epichlorohydrin (0.10 mol, 9.25 g) to a 100 mL single-necked bottle with magnets, heat up to 68 ° C, and add tetrabutyl dichloromethane after the raw materials are dissolved. Ammonium bromide (0.001 mol, 0.322 g). After 6 hours of reaction, a 15% sodium hydroxide aqueous solution (0.02 mol, 0.80 g) was added. After 3 hours of reaction, the reaction was stopped.60 mL of toluene was added and stirred for 10 minutes. The lower aqueous layer was discharged with a separatory funnel, and the organic phase was washed twice with water. The organic phase layer was collected and the solvent was spin-dried to obtain a crude product. The crude product was a mixed solvent of dichloromethane and petroleum ether (V : V = 3: 1) was used as the eluent for column separation to obtain 1.54 g of pure product with a yield of 82%.
References:
CN110407772,2019,A Location in patent:Paragraph 0053; 0063; 0067-0068
623-05-2
699 suppliers
$5.00/100mg

35198-42-6
19 suppliers
inquiry