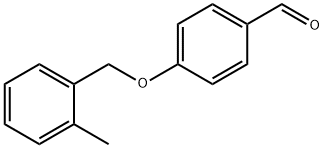
4-[(2-METHYLBENZYL)OXY]BENZENECARBALDEHYDE synthesis
- Product Name:4-[(2-METHYLBENZYL)OXY]BENZENECARBALDEHYDE
- CAS Number:400825-69-6
- Molecular formula:C15H14O2
- Molecular Weight:226.27
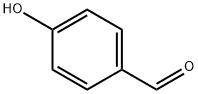
123-08-0
951 suppliers
$5.00/10g
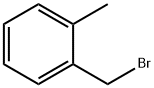
89-92-9
147 suppliers
$27.00/5g
![4-[(2-METHYLBENZYL)OXY]BENZENECARBALDEHYDE](/CAS/GIF/400825-69-6.gif)
400825-69-6
26 suppliers
$95.00/500mg
Yield:400825-69-6 100%
Reaction Conditions:
with caesium carbonate in N,N-dimethyl-formamide at 20; for 1 h;Inert atmosphere;
Steps:
20.A (20A) 4-[(2-Methylbenzyl)oxy]benzaldehyde
(20A) 4-[(2-Methylbenzyl)oxy]benzaldehyde 4-Hydroxybenzaldehyde (10.0 g, 81.9 mmol) and 2-methyl benzyl bromide (40.0 g, 98.3 mmol) were dissolved in dimethylformamide (100 mL), and cesium carbonate (18.2 g, 123 mmol) was added thereto, and then, the resulting mixture was stirred under a nitrogen atmosphere at room temperature for 1 hour. Water was added to the reaction solution, and the organic matter was extracted with diethyl ether. The organic layer was washed with water and a saturated sodium chloride solution, then dried over anhydrous magnesium sulfate and filtered. Then, the solvent was distilled off under reduced pressure, whereby a crude product was obtained. This crude product was purified by silica gel column chromatography (hexane:ethyl acetate=100:0 to 60:40 (v/v)), whereby the objective title compound was obtained as a colorless oily substance (19.2 g, yield: 103%). 1H NMR (CDCl3, 400 MHz): δ2.39 (3H, s), 5.13 (2H, s), 7.10 (2H, d, J=8.6 Hz), 7.21-7.31 (3H, m), 7.39 (1H, d, J=7.4 Hz), 7.86 (2H, d, J=8.6 Hz), 9.90 (1H, s)
References:
US2011/53974,2011,A1 Location in patent:Page/Page column 29