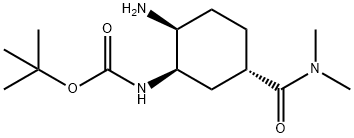
tert-butyl (1R,2S,5S)-2-aMino-5-(diMethylcarbaMoyl)cyclohexylcarbaMate synthesis
- Product Name:tert-butyl (1R,2S,5S)-2-aMino-5-(diMethylcarbaMoyl)cyclohexylcarbaMate
- CAS Number:365998-36-3
- Molecular formula:C14H27N3O3
- Molecular Weight:285.38
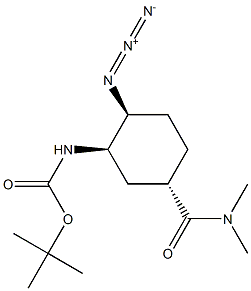
480450-69-9
37 suppliers
inquiry

365998-36-3
185 suppliers
$7.00/250mg
Yield:365998-36-3 87.7%
Reaction Conditions:
with methanol;palladium 10% on activated carbon;hydrogen at 50 - 60; under 3000.3 - 4500.45 Torr;Temperature;Reagent/catalyst;
Steps:
5-7 Example 6 Synthesis of tert-butyl N-[(1R,2S,5S)-2-amino-5-[(dimethylamino)carbonyl]cyclohexyl]carbamate
Add methanol (4B0g) to the hydrogenation kettle, add 109B8-01 (60g; 192.7mmol), then add 10% Pd/C (6.3g; water content about 56%);After stirring uniformly, blow in nitrogen to replace air 3 times; then blow in hydrogen to replace nitrogen 3 times; keep the pressure in the kettle at 0.4-0.6MPa, heat to 50-60°C; stir the reaction until no hydrogen is absorbed.After the reaction is completed, release the pressure, replace the hydrogen in the kettle with nitrogen, transfer the reaction liquid out of the reaction kettle; filter;Collect the filtrate, concentrate the dry solvent under reduced pressure to obtain a residue; dissolve the obtained residue with acetonitrile, filter out the insoluble matter, collect the filtrate, and then concentrate to dryness under reduced pressure, add toluene to the residue, heat to disperse, and cool to 05 Crystallize at , filter,The solid was collected and dried to obtain 109B9-01 with a dry weight of about 48.2 g. Yield: 87.7% (theoretical amount: 54.99 g).
References:
Inner Mongolia Jingdong Pharmaceutical Co., Ltd.;Lv Guanfeng;Xiao Jiang;Guo Rongyao CN111606827, 2020, A Location in patent:Paragraph 0059-0069
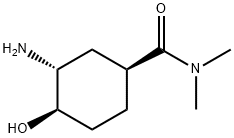
929693-36-7
16 suppliers
inquiry

365998-36-3
185 suppliers
$7.00/250mg
![(1S,3S,6R)-N,N-dimethyl-7-oxabicyclo[4.1.0]heptane-3-carboxamide](/CAS/20180527/GIF/929693-35-6.gif)
929693-35-6
37 suppliers
$65.00/1g

365998-36-3
185 suppliers
$7.00/250mg
![carbamic acid, n-[(1r,2r,5s)-5-[(dimethylamino)carbonyl]-2-hydroxycyclohexyl]-, 1,1-dimethylethyl ester](/CAS/20200331/GIF/929693-30-1.gif)
929693-30-1
68 suppliers
$110.00/500mg

365998-36-3
185 suppliers
$7.00/250mg
![(1R,2R,4S)-2-[(tert-butoxycarbonyl)aMino]-4-[(diMethylaMino)carbonyl]cyclohexyl Methanesulfonate](/CAS/20150408/GIF/929693-31-2.gif)
929693-31-2
54 suppliers
inquiry

365998-36-3
185 suppliers
$7.00/250mg