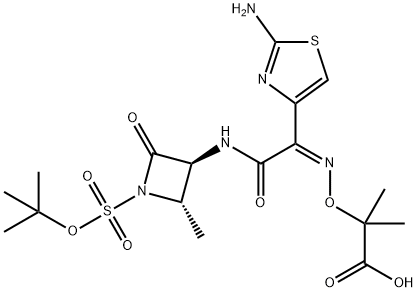
Propanoic acid, 2-[[(Z)-[1-(2-amino-4-thiazolyl)-2-[[(2S,3S)-2-methyl-4-oxo-1-sulfo-3-azetidinyl]amino]-2-oxoethylidene]amino]oxy]-2-methyl-, 1-(1,1-dimethylethyl) ester synthesis
- Product Name:Propanoic acid, 2-[[(Z)-[1-(2-amino-4-thiazolyl)-2-[[(2S,3S)-2-methyl-4-oxo-1-sulfo-3-azetidinyl]amino]-2-oxoethylidene]amino]oxy]-2-methyl-, 1-(1,1-dimethylethyl) ester
- CAS Number:330944-50-8
- Molecular formula:C17H25N5O8S2
- Molecular Weight:491.54

80082-65-1
241 suppliers
$6.00/1g
112941-26-1
150 suppliers
$9.00/25g
![Propanoic acid, 2-[[(Z)-[1-(2-amino-4-thiazolyl)-2-[[(2S,3S)-2-methyl-4-oxo-1-sulfo-3-azetidinyl]amino]-2-oxoethylidene]amino]oxy]-2-methyl-, 1-(1,1-dimethylethyl) ester](/CAS/20200515/GIF/330944-50-8.gif)
330944-50-8
15 suppliers
inquiry
Yield:-
Reaction Conditions:
Stage #1: (3S-trans)-3-Amino-4-methyl-2-oxo-1-azetidinesulfonic acid;(Z)-2-<<<1-(2-amino-4-thiazolyl)-2-<(2-benzothiazolyl)thio>-2-oxoethylidene>amino>oxy>-2-methyl propanoic acid 1,1-dimethylethyl esterwith triethylamine in water at 15 - 20;
Stage #2: in water;ethyl acetate; pH=5.0;
Stage #3: with hydrogenchloride in water at 0 - 5; pH=2.0;
Steps:
To a suspension of Azetidine of formula III (25 g) in mixture of aq.acetone or aq.THF, triethylamine (29 mL) was added. To the reaction mass, TAEM of formula (IV) (75 g) was added and the reaction mass was stirred at 150C -2O0C till completion of reaction. After completion of reaction was added a mixture of ethyl acetate and water (1 :1) and pH adjusted to 5.0. The by products obtained were removed, and layers separated. Organic layer was again extracted with water. The pH of the aqueous layer was acidified to 2.0 with dil. HCl at 00C -50C. The solid obtained was filtered, washed with water and dried under vacuum at 4O0C to yield title compound in pure form (48 g)
References:
WO2007/83187,2007,A2 Location in patent:Page/Page column 12

80082-65-1
241 suppliers
$6.00/1g
![(Z)-2-Amino-alpha-[1-(tert-butoxycarbonyl)]-1-methylethoxyimino-4-thiazolacetic acid](/CAS/GIF/86299-47-0.gif)
86299-47-0
174 suppliers
$19.00/1g
![Propanoic acid, 2-[[(Z)-[1-(2-amino-4-thiazolyl)-2-[[(2S,3S)-2-methyl-4-oxo-1-sulfo-3-azetidinyl]amino]-2-oxoethylidene]amino]oxy]-2-methyl-, 1-(1,1-dimethylethyl) ester](/CAS/20200515/GIF/330944-50-8.gif)
330944-50-8
15 suppliers
inquiry
149-30-4
644 suppliers
$5.00/25g