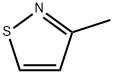
3-METHYLISOTHIAZOLE synthesis
- Product Name:3-METHYLISOTHIAZOLE
- CAS Number:693-92-5
- Molecular formula:C4H5NS
- Molecular Weight:99.15
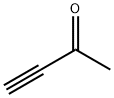
1423-60-5
179 suppliers
$23.00/1g

693-92-5
51 suppliers
$80.00/100mg
Yield: 40%
Reaction Conditions:
Stage #1:methyl ethynyl ketone with hydroxylamine-O-sulfonic acid in water at 0; for 0.5 h;
Stage #2: with sodium hydrogencarbonate in water at 0; for 0.333333 h;
Stage #3: with sodium hydrogen sulfide dihydrate in water at 0 - 20; for 16.25 h;
Steps:
Synthesis of 3-methylisothiazole (39)
To a stirred solution of but-3-yn-2-one 38 (17 g, 249.70 mmol) in H2O (100 mL) was added hydroxylamine-O-sulfonic acid (29.1 g, 257.23 mmol) at 0 oC and stirred for 30 min. To this was added sodium bicarbonate (23.72 g, 281.9 mmol) portion wise for 20 min at 0 oC, followed by dropwise addition of sodium hydrogen sulfide dihydrate (26 g, 282.2 mmol) in H2O (170 mL) at 0 oC for 15 min, then warming to room temperature and stirring for 16 h. The reaction was monitored by TLC. After completion of the reaction, the reaction mixture was diluted with water and extracted with diethyl ether. The combined organic extracts were dried over anhydrous sodium sulfate and concentrated in vacuo at 20 oC to afford compound 39 (10 g, 40%) as colorless syrup. TLC: 15% EtOAc/ hexanes (Rf: 0.5); 1H-NMR (DMSO- d6, 400 MHz): δ 8.96 (d, J = 4.5 Hz, 1H), 7.20 (d, J = 4.5 Hz, 1H), 2.45 (s, 3H).
References:
ASSEMBLY BIOSCIENCES, INC.;TURNER, William, W., Jr.;LI, Leping;HAYDAR, Simon, Nicolas;BURES, Mark, G.;RAI, Roopa;FRANCIS, Samson;ARNOLD, Lee, Daniel WO2018/160878, 2018, A1 Location in patent:Page/Page column 183
693-95-8
337 suppliers
$6.00/5g

693-92-5
51 suppliers
$80.00/100mg
66975-83-5
22 suppliers
$141.00/100 mg

693-92-5
51 suppliers
$80.00/100mg
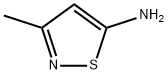
24340-76-9
51 suppliers
$192.00/1 g

693-92-5
51 suppliers
$80.00/100mg